A simple way to significantly increase lifetimes of fuel cells, other key devices
In research that could jumpstart work on a range of technologies including fuel cells, key to storing solar and wind energy, MIT researchers have found a relatively simple way to increase the lifetimes of these devices: changing the "pH" of the system.
Fuel and electrolysis cells made of materials known as solid metal oxides are of interest for several reasons. For example, in the electrolysis mode, they are very efficient at converting electricity from a renewable source into a storable fuel like hydrogen or methane that can be used in the fuel cell mode to generate electricity when the sun isn't shining or the wind isn't blowing. They can also be made without using costly metals like platinum. However, their commercial viability has been hampered, in part, because they degrade over time. Metal atoms seeping from the interconnects used to construct banks of fuel/electrolysis cells slowly poison the devices.
"What we've been able to demonstrate is that we can not only reverse that degradation, but actually enhance the performance above the initial value by controlling the acidity of the air electrode interface," says Harry L. Tuller, the R.P. Simmons Professor of Ceramics and Electronic Materials in MIT's Department of Materials Science and Engineering (DMSE).
The research, initially funded by the U.S. Department of Energy through the Office of Fossil Energy and Carbon Management's (FECM) National Energy Technology Laboratory, should help the Department meet its goal of significantly cutting the degradation rate of solid oxide fuel cells by 2035–2050.
"Extending the lifetime of solid oxide fuels cells helps deliver the low-cost, high-efficiency hydrogen production and power generation needed for a clean energy future," says Robert Schrecengost, Acting Director of FECM's Division of Hydrogen with Carbon Management. "The Department applauds these advancements to mature and ultimately commercialize these technologies so that we can provide clean and reliable energy for the American people."
"I've been working in this area my whole professional life, and what I've seen until now is mostly incremental improvements," says Tuller, who was recently named a 2022 Materials Research Society Fellow for his career-long work in solid-state chemistry and electrochemistry. "People are normally satisfied with seeing improvements by factors of 10's of percent. So, actually seeing much larger improvements and, as importantly, identifying the source of the problem and the means to work around it, issues that we've been struggling with for all these decades, is remarkable."
Says James M. LeBeau, another MIT professor involved in the work, "this work is important because it could overcome [some] of the limitations that have prevented the widespread use of solid oxide fuel cells. Additionally, the basic concept can be applied to many other materials used for applications in the energy-related field." LeBeau is the John Chipman Associate Professor of Materials Science and Engineering
The work was reported August 11, online, in Energy & Environmental Science. Additional authors of the paper are Han Gil Seo, a DMSE postdoctoral fellow; Anna Staerz, formerly a DMSE postdoctoral fellow, now at Interuniversity Microelectronics Center (IMEC) Belgium and soon to join the Colorado School of Mines faculty; Dennis S. Kim, a DMSE postdoctoral associate; Dino Klotz, a DMSE visiting scientist, now at Zurich Instruments; Michael Xu, a DMSE graduate student, and Clement Nicollet formerly a DMSE postdoctoral fellow, now at the Université de Nantes. Seo and Staerz contributed equally to the work.
What they did
A fuel/electrolysis cell has three principal parts: two electrodes (a cathode and anode) separated by an electrolyte. In the electrolysis mode, electricity from, say, the wind, can be used to generate storable fuel like methane or hydrogen. On the other hand, in the reverse fuel cell reaction, that storable fuel can be used to create electricity when the wind isn't blowing.
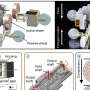
A working fuel/electrolysis cell is composed of many individual cells that are stacked together and connected by steel metal interconnects that include the element chrome to keep the metal from oxidizing. But "it turns out that at the high temperatures that these cells run, some of that chrome evaporates and migrates to the interface between the cathode and the electrolyte, poisoning the oxygen incorporation reaction," Tuller says. After a certain point, the efficiency of the cell has dropped to a point where it is not worth operating any longer.
"So if you can extend the life of the fuel/electrolysis cell by slowing down this process, or ideally reversing it, you could go a long way towards making it practical," Tuller says.
The team showed that you can do both by controlling the acidity of the cathode surface. They also explained what is happening.
Changing the acidity
To achieve their results, the team coated the fuel/electrolysis cell cathode with lithium oxide, a compound that changes the relative acidity of the surface from being acidic to being more basic. "After adding a small amount of lithium, we were able to recover the initial performance of a poisoned cell," Tuller says. When the engineers added even more lithium, the performance improved far beyond the initial value. "We saw improvements of three to four orders of magnitude in the key oxygen reduction reaction rate and attribute the change to populating the surface of the electrode with electrons needed to drive the oxygen incorporation reaction."
The engineers went on to explain what is happening by literally observing the material at the nanoscale, or billionths of a meter, with state-of-the-art transmission electron microscopy and electron energy loss spectroscopy. "We were interested in understanding the distribution of the different chemical additives [chromium and lithium oxide] on the surface," says LeBeau.
They found that the lithium oxide effectively dissolves the chromium to form a glassy material that no longer serves to degrade the cathode performance.
What's next?
Many technologies like fuel cells are based on the ability of the oxide solids to rapidly breathe oxygen in and out of their crystalline structures, Tuller says. The MIT work essentially shows how to recover—and speed up—that ability by changing the surface acidity. As a result, the engineers are optimistic that the work could be applied to other technologies including, for example, sensors, catalysts, and oxygen permeation-based reactors.
The team is also exploring the effect of acidity on systems poisoned by different elements, like silica.
Concludes Tuller: "As is often the case in science, you stumble across something and notice an important trend that was not appreciated previously. Then you test that concept further, and you discover that it is really very fundamental."
More information: Han Gil Seo et al, Reactivation of chromia poisoned oxygen exchange kinetics in mixed conducting solid oxide fuel cell electrodes by serial infiltration of lithia, Energy & Environmental Science (2022). DOI: 10.1039/D1EE03975J