Transition metal composite provides a cost-effective boost for lackluster zinc–air battery performance
Rechargeable zinc–air batteries, powered by oxidizing zinc with oxygen from the air, offer an efficient storage option for renewable energy that's both clean and safe. Battery performance, however, has been hampered by slow oxygen electrochemical reactions, a critical bottleneck for scaling and commercializing.
In their study published in Particuology, a team of researchers in China designed a strategy to improve battery performance that involves boosting oxygen reactions by combining two transition metals to deliver high electrocatalytic activity.
Most renewable energy sources, including solar energy, lack long-term stability and require high-efficient energy storage systems to integrate with the electric grid. Rechargeable zinc–air batteries are considered good candidates for next-generation energy storage because they pack a punch with ultrahigh theoretical energy density. These batteries draw one of their main reactants, oxygen, from the air. They contain no toxic compounds and can be recycled, safely disposed of, and recharged with new zinc.
The hurdle lies in a pair of electrochemical reactions—oxygen evolution reaction (OER) and oxygen reduction reaction (ORR)—that take place at the air cathode during battery charge and discharge.
"The redox kinetics for ORR and OER are highly sluggish and render severe polarization, decreased energy efficiency, and limited cycling lifespan of practical rechargeable zinc–air batteries," said paper author Bo-Quan Li, associate professor at the Beijing Institute of Technology.
For zinc-air batteries to be viable on the large scale, these reactions need a boost. Noble metals and transition metals (nickel, cobalt, manganese, and iron) can be used to catalyze ORR and OER kinetics by, for example, accelerating the transfer of electrons between electrode and reactants. These techniques work, but at a high cost.
"Noble-metal-based electrocatalysts demonstrate state-of-the-art electrocatalytic activity and serve as widely accepted benchmarks," said Li. "But the high cost, earth scarcity, and poor durability hinder their large-scale practical applications."
As such, the ongoing search for a high performance noble-metal-free option that catalyzes both ORR/OER is of great importance for practical rechargeable zinc–air batteries, said Li.
Previous studies have shown that embedding transition metal atoms into a conductive carbon substrate produces high electrocatalytic activity due to atomic efficiency, unique electronic structure, and diversity in chemical structure. But which metal works best for both ORR and OER?
In their study, the research team from Beijing Institute of Technology, Tsinghua University and Harbin Normal University ask: why choose just one?
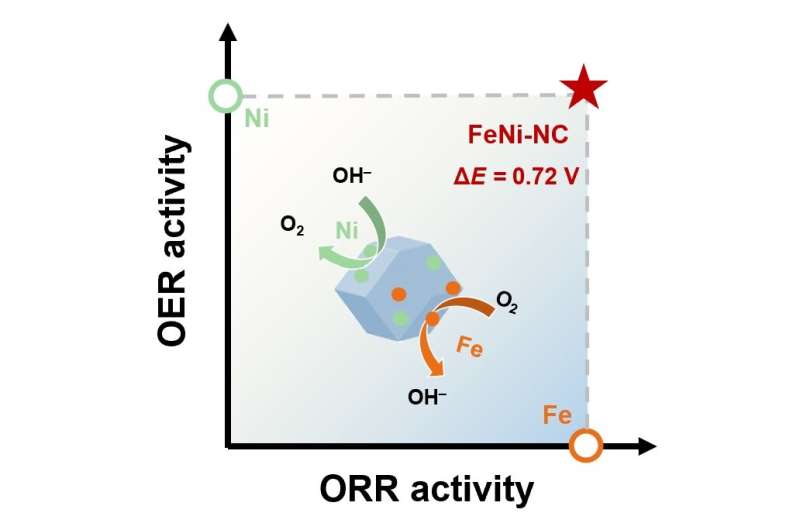
"A single type of active site can hardly promote both ORR and OER kinetics simultaneously to provide outstanding bifunctional electrocatalytic activity," said Li. "Composing different active sites with respective electrocatalytic activity has been verified as an effective strategy to realize multi-functionality."
The research team combined two atomic transition metal sites—atomic iron (Fe) and atomic nickel (Ni)—and embedded the composite over a nitrogen-doped carbon (NC) substrate. Fe achieved high electrocatalytic activity on oxygen reduction, while Ni successfully boosted oxygen evolution. Together, they realized high-active electrocatalysts in both reactions.
"The composite electrocatalyst demonstrated outstanding bifunctional electrocatalytic activity that surpasses the noble-metal-based electrocatalyst and most of the reported bifunctional electrocatalysts based on analogous active sites," said Li.
Researchers showed that rechargeable zinc–air batteries equipped with the FeNi-NC electrocatalyst achieved high peak power density, high working rates, and long lifespan.
In addition to effectively boosting battery performance, Fe and Ni are cost-effective, scalable alternatives to the more expensive and scarcer noble metal oxygen electrocatalysts in rechargeable zinc–air batteries.
The research team is now developing techniques to optimize the configuration of the atomic transition metal sites and promote the cycling stability under working conditions.
"The ultimate goal is to realize high-rate, high-capacity, and long-cycling rechargeable zinc–air batteries for practical applications," said Li.
More information: Juan Wang et al, Composing atomic transition metal sites for high-performance bifunctional oxygen electrocatalysis in rechargeable zinc–air batteries, Particuology (2022). DOI: 10.1016/j.partic.2022.09.003