March 9, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New direct air capture device three times more efficient than current approaches
A team of environmental engineers at Lehigh University working with a colleague from Georgia Tech Shenzhen Institute has developed a direct air capture (DAC) device for removing CO2 from the air. The results are published in the journal Science Advances.
As climate change progresses, scientists around the world are working to prevent global disaster. Some see ways to reduce gas emissions, while others note that so much carbon has already been released into the atmosphere that simply reducing emissions now is not enough—some means for removing it must be found. Such approaches revolve around the design and creation of DAC devices.
Most of those currently in use involve forcing air through sorbent filters that bind with CO2. In this approach, the filters must be heated to allow for binding reactions to occur—they also need to work in a vacuum. Once the CO2 is captured, it must then be compressed and stored. However, the Industrial Emissions Directive reported in 2022 that all such facilities together now operating in the world collect approximately 0.01 million metric tons of CO2 per year. But to make a dent in the CO2 in the air, they estimate that approximately 85 million metric tons must be removed each year. Clearly, a better approach is needed.
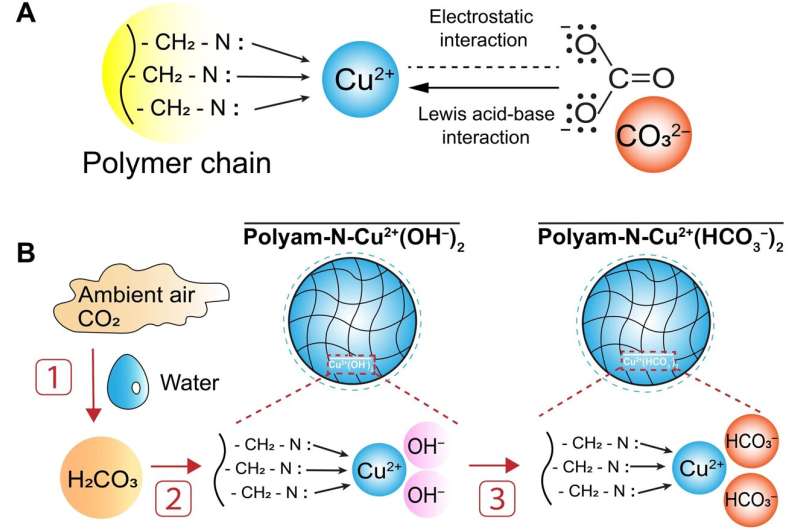
To improve efficiency, the team in Pennsylvania designed a new kind of sorbent made of synthetic resin that, when dipped in copper-chloride solution, forces the CO2 in the air to bind to the resin. This makes the binding reaction run faster and also uses less energy than other designs. The researchers note that the sorbent can also be regenerated using salt solutions. Perhaps more importantly, their approach results in the production of carbonic acid rather than carbon, which, they note, can be made easily into baking soda. They even suggest that the baking soda could be dumped into the ocean, where it will not cause any harm.
Testing showed that the approach is capable of absorbing up to three times as much CO2 as conventional systems—not nearly enough to make a difference on a global scale, but a clear starting point for new avenues of research.
More information: Hao Chen et al, Direct air capture (DAC) and sequestration of CO 2 : Dramatic effect of coordinated Cu(II) onto a chelating weak base ion exchanger, Science Advances (2023). DOI: 10.1126/sciadv.adg1956
© 2023 Science X Network