This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Slow electrons for more efficient reactions
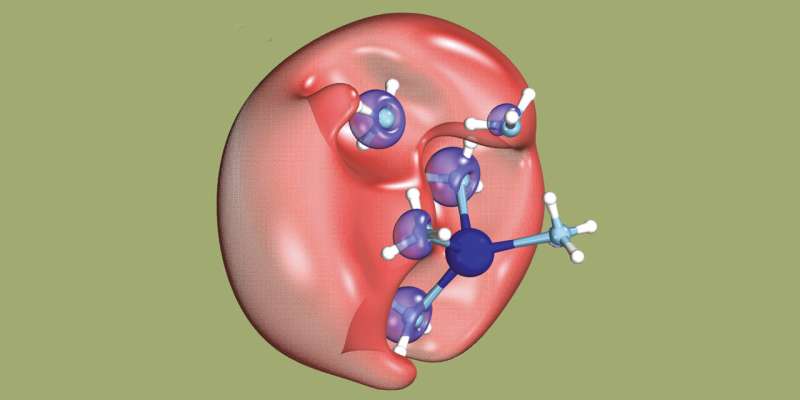
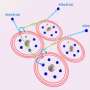
What an international team of researchers actually set out to do was to detect a mysterious chemical object: a dielectron in solution. A dielectron is made up of two electrons, but unlike an atom, it has no nucleus. Up to now, scientists have been unable to directly detect such an object. While the researchers led by ETH Zurich Professor Ruth Signorell were experimenting with dielectrons, they accidentally discovered a new process for producing slow electrons. These can be used to initiate certain chemical reactions.
Dielectrons are unstable. They break apart again into two electrons in less than one-trillionth of a second. As the researchers were able to show, one of these electrons remains in place, while the other—which has low energy and is therefore relatively slow—moves away. What's special about the new method is that it allows the researchers to control the kinetic energy of this electron and thus its speed.
Dielectrons occupy cavities
But first things first: to produce the dielectrons, the researchers dissolved sodium in (liquid) ammonia and exposed this solution to UV light. This exposure causes an electron from an ammonia molecule to join an electron from a sodium atom and thus form a dielectron. The dielectron briefly occupies a tiny cavity in the solution. The researchers managed to show that when the dielectron breaks up, one of the electrons moves away at a speed determined by the wavelength of the UV light used. "Some of the UV light energy has been transferred to the electron," Signorell says.
For their study now published in Science, The ETH Zurich researchers carried out this work in collaboration with researchers from the University of Freiburg in Germany, the SOLEIL synchrotron in France and Auburn University in the United States.
Examining reactions and radiation damage
Such electrons with low kinetic energy are interesting for a variety of reasons. One is that slow electrons cause radiation damage to human tissue. Mobile electrons can form in this tissue, for instance as a result of X-rays or radioactivity. They can then attach to DNA molecules and trigger chemical reactions. Producing such slow electrons more easily in the lab will help researchers better examine the mechanisms that lead to radiation damage.
But the human body isn't the only place chemical reactions are induced by a compound accepting a free electron. The production of synthetic cortisone and other steroids is just one example.
Making it possible to use UV light as a relatively simple means of producing slow electrons directly in a solution, and also controlling the energy of the electron, will make it easier to better examine these reactions in the future. It may even be possible for chemists to optimize reactions, for example by using UV light to increase the electrons' kinetic energy.
More information: Sebastian Hartweg et al, Solvated dielectrons from optical excitation: An effective source of low-energy electrons, Science (2023). DOI: 10.1126/science.adh0184