This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers demonstrate the functioning of a photo-rechargeable two electrode battery
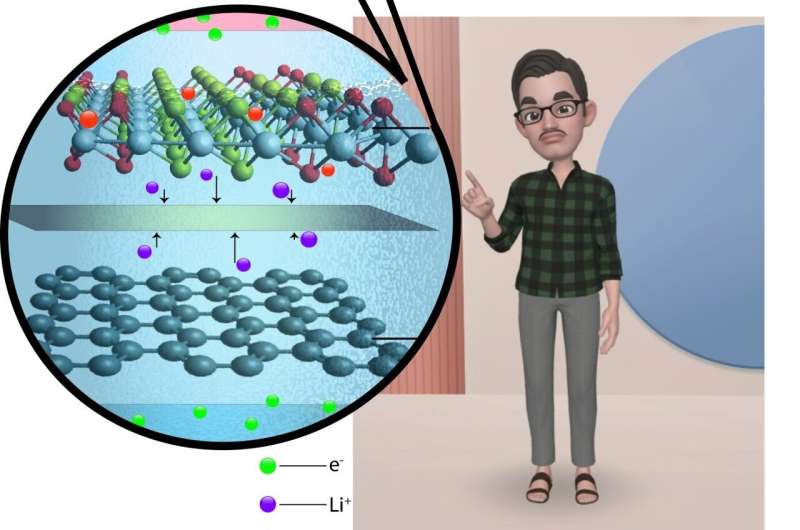
T. N. Narayanan and colleagues from the Tata Institute of Fundamental Research, Hyderabad (TIFRH) report their recent findings on how a lithium ion battery, similar to existing commercial designs, can be directly charged using light.
In 1976, Stanley Whittingham accelerated advancements in the field of energy storage by successfully demonstrating the first proof-of-concept rechargeable lithium ion battery. This design used a layered material titanium disulfide (TiS2) as a cathode and lithium as an anode.
The usage of lithium metal as an anode raised security concerns, as a result of which scientists went back to the drawing board to come up with a commercially viable design. In 1985, Akira Yoshino showed that carbon can replace the lithium metal, leading to the commercialization of the first lithium ion battery by Sony Energy Devices Corporation in 1991.
This design was improved upon by John B. Goodenough, who suggested a change from TiS2 to cobalt oxide to function as a cathode. Whittingham, Yoshino and Goodenough were jointly awarded the Nobel Prize in Chemistry in 2019 for their pioneering research towards developing lithium ion batteries that has revolutionized modern day energy-storage.
Scientists have continued their efforts towards developing safer and durable batteries that can be recharged easily. Some of these advancements include experimenting with different materials of electrodes. Michael de Volder's lab at University of Cambridge, were the first to report the use of polycrystalline metal-halide based 2D perovskite materials in a photo-rechargeable battery.
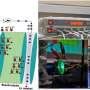
Three years later, T. N. Narayanan's group used a heterostructure (combination of two materials molybdenum sulfide and molybdenum oxide) electrode in a lithium ion battery. It was in this study that Narayanan and colleagues observed a charging mechanism driven by light. Amar Kumar, lead author and graduate student at T. N. Narayanan's group, explored whether whether light was indeed charging the battery or was this observation simply a result of an unknown side reaction.
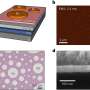
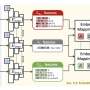
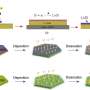
Recently, Kumar and colleagues were able to successfully demonstrate the functioning of a light chargeable battery with lithium metal-TiS2/TiO2 hybrid electrode (half-cell) assembly. The TIFRH team further improved this design by replacing lithium with graphite, thus, developing a safer solar battery.
Lithium intercalated TiS2/TiO2 as cathode and graphite as an anode seem to be functioning as a battery, with an efficiency similar to what is used in cell phones, but are also rechargeable using light with solid electrolytes—hence, safer batteries. Computational studies providing further mechanistic insights were conducted by Soumya Ghosh's group at TIFR Hyderabad.
This work opens up a plethora of opportunities for the development of commercially viable light chargeable batteries while also raising several fundamental questions regarding charging mechanism, thermal effects etc. Presently, T. N. Narayanan's group (TIFR Hyderabad) in collaboration with Michael De Volder's group (University of Cambridge) are exploring potential candidate materials for developing robust renewable energy harvesting cum storage systems.
The researchers are also trying to obtain a clearer understanding of the mechanism driving the functioning of such candidate systems.
The findings are published in the journal Small.
More information: Amar Kumar et al, Photo‐Rechargeable Li‐Ion Batteries using TiS2 Cathode, Small (2023). DOI: 10.1002/smll.202303319