This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A potentially cheaper and 'cooler' way to transport hydrogen
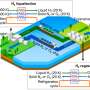
In the continued effort to move humanity away from fossil fuels and towards more environmentally friendly energy sources, researchers in Japan have developed a new material capable storing hydrogen energy in a more efficient and cheaper manner.
The new hydrogen energy carrier can even store said energy for up to three months at room temperature. Moreover, since the material is nickel based, its cost is relatively cheap. The results were reported in Chemistry—A European Journal.
As humanity combats the ongoing climate crisis, one avenue researchers focus on is the transition into alternative sources of energy such as hydrogen. For several decades now Kyushu University has been investigating ways to more efficiently use and store hydrogen energy in the effort to realize a carbon neutral society.
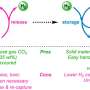
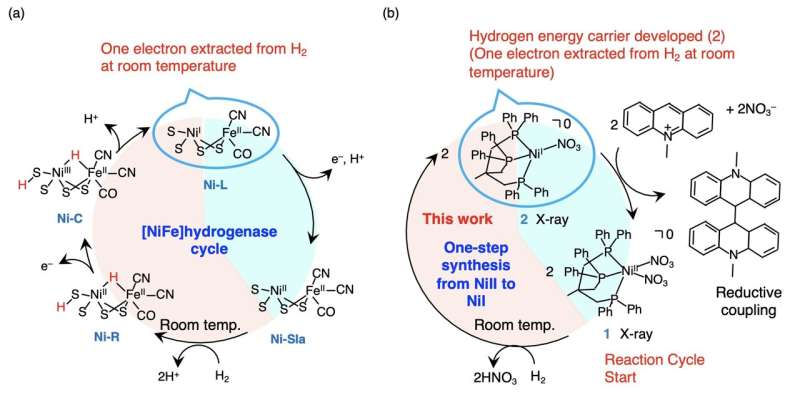
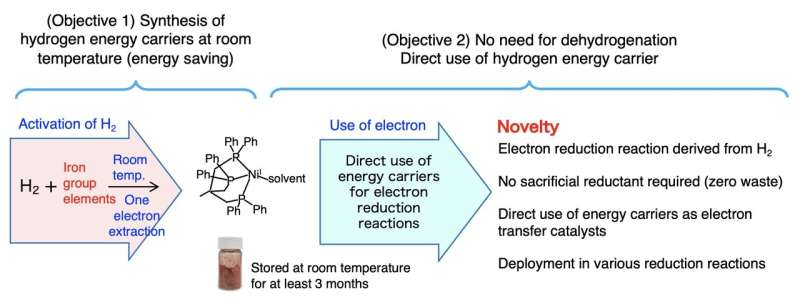
"We have been working on developing new materials that can store and transport hydrogen energy," explains Professor Seiji Ogo of Kyushu University's International Institute for Carbon-Neutral Energy Research who led the research team. "Transporting it in its gaseous state requires significant energy. An alternative way of storing and transporting it would be to 'split-up' the hydrogen atoms into its base components, electrons and protons."
Many candidates have been considered as possible hydrogen energy carries such as ammonia, formic acid, and metal hydrides. However, the final energy carrier had not yet been established.
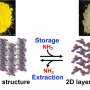
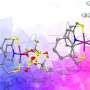
"So, we looked to nature for hints. There are a series of enzymes called hydrogenases that catalyze hydrogen into protons and electrons and can store that energy for later use, even at room temperature," continues Ogo. "By studying these enzymes our team was able to develop a new compound that does exactly that."
Not only was their new compound able to extract and store electrons at room temperature, further investigations showed that it can be its own catalyst to extract said electron, something that had not been possible with previous hydrogen energy carriers. The team also showed that the energy could be stored for up the three months.
Ogo also highlights the fact that the compound uses an inexpensive element: nickel. Until now, similar catalysts have used expensive metals like platinum, rhodium, or iridium. Now that nickel is a viable option for hydrogen energy storage, it can potentially reduce the cost of future compounds.
The team intends to collaborate with the industrial sector to transfer their new findings into more practical applications.
"We would also like to work on improving storage time and efficiency as well as investigate the viability of cheaper metals for such compounds," concludes Ogo. "Hopefully our findings will contribute to the goal of decarbonization so that we can build a greener and environmentally friendly future."
More information: Chiaki Takahashi et al, Single‐Step Synthesis of NiI from NiII with H2, Chemistry—A European Journal (2023). DOI: 10.1002/chem.202302297