This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How a small 'gap' brings green energy storage through electrolysis closer
Green hydrogen is a serious contender to become a renewable energy carrier. For example, we could use it to store solar and wind energy during peaks in order to utilize the energy when the sun is no longer shining and the wind is calm. This would require more electrolyzers that can efficiently and flexibly handle fluctuations in power supply.
However, the electrolyzers that can already do so use rare and expensive raw materials for their electrodes, such as platinum and iridium, while more affordable alkaline electrolyzers struggle to operate flexibly. This is where a team of TU/e researchers, led by Thijs de Groot (Chemical Engineering & Chemistry), may have now found a solution.
In the search for viable solutions in the energy transition, the focus is not just on finding sustainable energy sources. In fact, solutions that enable us to efficiently store energy are crucial to managing the peaks and troughs in energy supply and demand.
One of the solutions being explored is hydrogen. This can be produced relatively easily from water through electrolysis and can then be stored or used as a fuel or raw material for industry.
Unsurprisingly, a lot of research is being done on electrolysis, especially on how to make this technique affordable, sustainable, and suitable for dealing with a highly variable supply of electricity. At TU/e, we are also doing a lot of research on this, as described in a clear overview from 2021.
As that article explains, sustainable and affordable alkaline electrolyzers find it tricky to cope with a flexible supply of electricity. No wonder that de Groot—who recently started working full-time at TU/e as an associate professor of Sustainable Process Engineering—and his team have focused their attention on this very issue.
"You can make alkaline electrolyzers with relatively cheap raw materials, which makes them very suitable as a sustainable choice. That's exactly why I wanted to do research on them—to see if we can make them suitable for flexible energy storage," says de Groot.
The challenge: The hydrogen leak
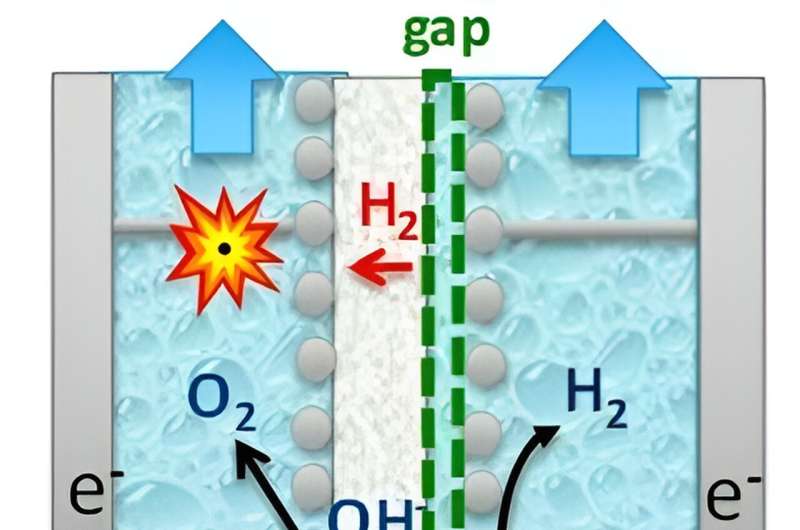
So, what's the most difficult thing about these alkaline electrolyzers? To answer this, we have to go back to the basics of what happens in an electrolyzer, where a cathode and an anode are placed in a salt solution with a membrane between them. If you apply enough voltage, a current flows; hydrogen (H2) forms at the cathode and oxygen (O2) at the anode.
The membrane is supposed to keep the hydrogen and oxygen from coming together. However, no membrane is able to keep oxygen and hydrogen perfectly apart, which can potentially create dangerous situations.
The danger lies in the hydrogen leaking through the membrane and ending up on the oxygen side (known as hydrogen crossover). If the amount of hydrogen in the oxygen exceeds 4%, an explosive mixture is created.
Hydrogen leakage always takes place to some extent but is especially dangerous when the electrolyzer is not running at full power. In that case, less oxygen is made, so the hydrogen leaking through the membrane is not diluted as strongly. The concentration then approaches the explosive limit more quickly.
To mitigate this danger, the preference is now for alkaline electrolyzers to run at full power. They can be turned up or down a bit but cannot handle sudden large changes. This makes it more difficult to connect them directly to solar or wind farms.
It is also tough to operate the electrolyzers with hydrogen at very high pressure because even more hydrogen will then leak through the membrane. But perhaps a change is coming.
The solution: Managing the gap
To enhance the flexibility of alkaline electrolyzers and ensure their safe operation when connected to variable power supply, we aim to control the hydrogen crossover. The distance between the membrane and the cathode—the gap—is very important to this.
In the past, research has been done on these gaps at electrodes. Those studies focused mainly on the efficiency of electrolysis. And, this efficiency is greatest with a zero gap at the cathode.
However, if there is no gap, a lot of gas will move through the membrane. This is due to a high supersaturation of hydrogen near the membrane. Supersaturation is the same phenomenon that occurs when you open a bottle of beer or a carbonated soft drink. So, this supersaturation must be brought down if you want to make your electrolyzer more flexible without the danger of an explosion.
De Groot states, "For this reason, Rodrigo Lira Garcia Barros—Ph.D. student and first author of the article—systematically investigated the effect of gap size during his doctoral research. This concerned, in particular, the effect on the amount of hydrogen moving through the membrane and the performance of the electrolyzer."
Lira Garcia Barros and de Groot supervised the master's student Joost Kraakman, who developed a model for hydrogen crossover and also designed the research set-up. Most of the measurements were then performed by bachelor's student Carlijn Sebrechts. The findings are published in the International Journal of Hydrogen Energy.
"It then turned out that with a small but measurable gap at the cathode, we can greatly reduce the leakage of hydrogen through the membrane—and with an acceptable loss in performance! In the end, you're left with a more functional and, above all, more flexible electrolyzer," says de Groot.
"That's the conclusion of our scientific analysis, so it's now time for the next step: creating a proof of concept. That's what we're going to do in our lab, through which we expect to be able to prove that a flexible electrolyzer is scalable to industry."
Scalable electrolyzers can also be made in different sizes. For example, very large ones could be used for central energy storage, just as power plants now operate centrally in our grid, or smaller ones could accommodate peaks in power generation at the neighborhood or wind farm level.
"It should be possible to design alkaline electrolyzers that have a higher load flexibility using existing commercial electrodes and diaphragms," concludes Lira Garcia Barros.
The next step: High-pressure electrolysis
De Groot enthusiastically goes on to talk about a new European project that is also starting at TU/e: high-pressure electrolysis. "The hydrogen released in electrolysis is a gas. But gas can only be used, stored, and transported efficiently if it is stored at a sufficiently high pressure."
"We currently need a compressor for that, in addition to the electrolyzer itself. Those devices are expensive, noisy, and unreliable. So, what if we could make an electrolyzer that immediately delivers the hydrogen at a high pressure?"
"A few years back, I thought that this wouldn't be possible, just as I thought that we wouldn't be able to make a super-flexible alkaline electrolyzer. We managed to do the latter, so I'm very much looking forward to again working with students on this new challenge."
More information: Rodrigo Lira Garcia Barros et al, Impact of an electrode-diaphragm gap on diffusive hydrogen crossover in alkaline water electrolysis, International Journal of Hydrogen Energy (2023). DOI: 10.1016/j.ijhydene.2023.09.280