This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Strategies to limit redox electrolyte-enhanced carbon-based supercapacitor self-discharge
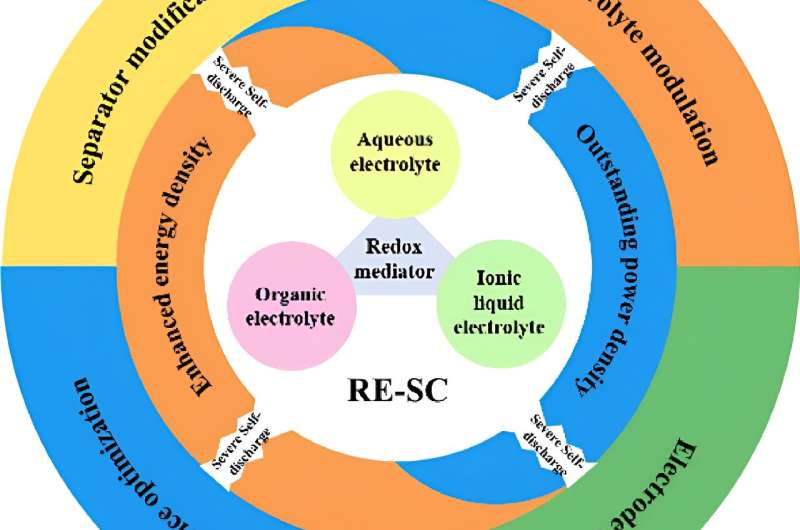
The efficient storage of clean energy is a critical component to achieving carbon neutrality. Capacitors are devices that store energy by separating positive and negative electrical charge, and supercapacitors (SC) are capacitors that can store and release large amounts of energy. A specialized supercapacitor, called a redox electrolyte-enhanced SC (RE-SC), places liquid redox electrolytes, a source of ions that can be electrically charged, next to a carbon-based electrical conductor, or electrode, to achieve high energy storage density and power output.
Despite this increase in performance, RE-SCs suffer from self-discharge of stored energy, limiting their practicality. Research is underway to develop self-discharge suppression strategies that will improve RE-SC performance and clean energy storage efficiency.
Current energy storage technologies suffer from a litany of tradeoffs: for example, high energy-density batteries do not achieve a power output high enough to meet demand and SCs supply enough power to meet demand but suffer from low energy density. Likewise, RE-SCs improve energy densities compared to traditional SCs but rapidly self-discharge.
Despite the disadvantages associated with self-discharge, RE-SCs have many advantages, including easy redox electrolyte and electrode preparation, scale up and assembly, long life and simple performance customization. Researchers are investigating new ways to overcome self-discharge in RE-SCs, including separator modification, electrolyte modulation, electrode design and overall device optimization.
A team of materials scientists from the Chinese Academy of Sciences and North University of China in Taiyuan and the University of Chinese Academy of Sciences in Beijing have published a review on the current state of RE-SC research and challenges the field has to overcome in Energy Materials and Devices.
"The charged SC undergoes a spontaneous voltage drop under an open circuit, which, similar to Achilles heel, limits the wide application of the capacitor. The energy loss depends on the self-discharge rate, which varies for different types of redox-active electrolyte species used in the device. To greatly diminish the harmful effects of self-discharge, it is necessary to understand the mechanism of self-discharge…. Ohmic leakage, Faraday reaction and charge redistribution are perceived as three widely accepted mechanisms of self-discharge in RE-SCs," said Xiaodong Tian, an author of the review paper and researcher in the CAS Key Laboratory for Carbon Materials at the Chinese Academy of Sciences and the Center of Materials Science and Optoelectronics Engineering at the University of Chinese Academy of Sciences.
Specifically, charge redistribution refers to the movement of charge down a concentration gradient, from higher concentration to lower concentration, after the external power supply is removed. This aspect of self-discharge results in a lower voltage and lower energy storage in the RE-SC. Importantly, optimizing the pore size distribution (PSD) of the electrode could change the diffusion of ions in the RE-SC, ameliorating self-discharge due to this phenomenon.
"In contrast, Ohmic leakage is typically derived from an internal short circuit or parasitic redox reactions near the electrode/electrolyte interface. The Faraday reaction originates from the reactants, such as oxygen-functional groups on the carbon electrode surface, redox mediator and dissolved oxygen in the electrolyte, that can accept or lose electrons," said Tian.
To overcome these factors, materials scientists are investigating several promising strategies. One strategy is to modify the separator in the RE-SC. In a SC, the separator insulates or shields the negative electrode from the positive electrode and provides channels that serve as openings for ions to move back and forth to store and discharge energy. Researchers argue that optimizing the pore size, ion selectivity and polarity of the separator could provide one means of limiting RE-SC self-discharge. Ion exchange membranes, for example, have already reduced self-discharge in RE-SCs but are currently too cost-prohibitive for wide-spread use.
Optimization of the redox electrolyte could also decrease self-discharge. Some electrolytes can form reversibly solid complexes during charging and recharging, achieving high energy density, power output, long life and slow self-discharge rates. Additionally, ionic liquids can be designed with faster redox kinetics to improve power output and cycling life compared to immobilized redox species.
The pore structure and surface polarity of RE-SC electrodes are another critical component for limiting self-discharge. Previous research performed on separator and electrolyte optimization suggests that strengthening the interaction between ions and the electrode surface will limit the amount of self-discharge of the entire RE-SC system. For example, carbon-based electrodes with pore sizes of less than 1 nm (10-9 m) demonstrate the slower self-discharge. The addition of a functional barrier layer and asymmetric designs that employ more than one electrode or electrolyte might also decrease self-discharge.
The research team is excited by the potential of RE-SCs but acknowledges that significant hurdles must be overcome before RE-SCs become a practical means of energy storage. "Compared with the conventional electrical double-layer capacitors, the research on the RE-SC systems is still at an infant stage. Extensive and thorough investigations are desired for their applications. Therefore, more attention should be paid to alleviating the severe self-discharge concern in RE-SCs," said Tian.
More information: Jiyong Shi et al, Redox electrolyte-enhanced carbon-based supercapacitors: recent advances and future perspectives, Energy Materials and Devices (2023). DOI: 10.26599/EMD.2023.9370009