January 15, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Model outlines how ionic blockades influence energy recovery in forward bias bipolar membranes
Bipolar membranes (BPMs) are a class of ion-exchange membranes typically comprising a cation- and an anion-exchange layer. While these membranes have recently been integrated in various electrochemical devices for a wide range of application, the processes underlying their operation are not yet fully understood.
Researchers at the Massachusetts Institute of Technology (MIT) recently developed a new mechanistic model that explains the forward bias polarization mechanisms of BPMs in mixed electrolytes with varying acidities and basicities. Their model, introduced in Nature Energy, could guide the development of strategies to overcome the issue of ionic blockades, which can adversely impact the performance of forward bias BPM devices.
"We were initially trying to design an electrolyzer that converts carbon dioxide CO2 into useful feedstocks or fuels using bipolar membranes (BPMs)," Yogesh Surendranath, co-author of the paper, told Tech Xplore. "To provide a little context, CO2 electrolyzers are most efficient when operating with alkaline electrolyte solutions such as potassium hydroxide, but because CO2 is an acid gas, it reacts with alkaline solutions to produce carbonate solutions over time."
The chemical reactions that can occur when CO2 electrolyzers operate with alkaline solutions significantly impact these technologies' efficiency, thus preventing their widespread and long-term adoption. In fact, these reactions cause CO2 feedstock to migrate and escape into a different part of the electrolyzer, while also causing the employed electrolyte solutions to become less alkaline.
"We envisioned that with forward bias BPMs, we could engineer an electrolyzer that would take the carbonated electrolyte and react it in an acid-base reaction in the BPM to reform CO2 gas," Surendranath explained. "This would in turn allow us to build an electrolyzer that is stable and viable in the long term."
While they were working on the electrolyzer they envisioned, Surendranath and his colleagues realized that the electrochemistry of forward bias BPMs was still poorly understood. They thus set out to fill some gaps in the literature, which would ultimately allow them to realize their electrolyzer.
"Once we had conducted a few preliminary experiments to shed some light on how forward bias BPMs operated, we realized that we had an opportunity to undertake a much more interesting study to uncover the mechanism of forward bias BPMs, and decided to dive headfirst into this effort," Wei Lun Toh, co-author of the paper, told Tech Xplore.
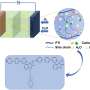
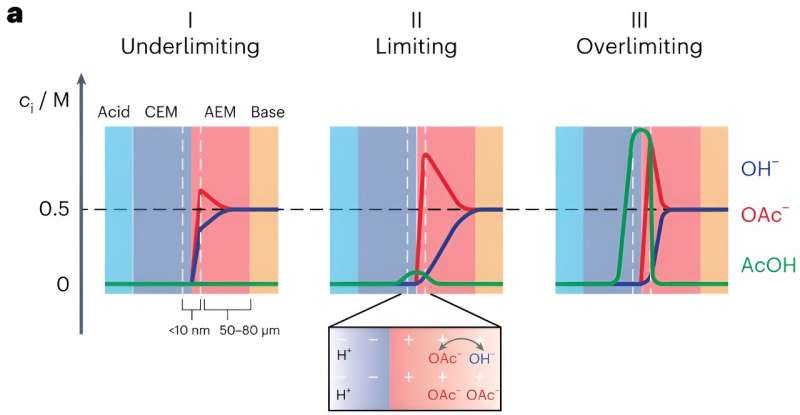
A BPM is essentially made up of a positively charged membrane that transports anions (i.e., the anion-exchange membrane) and a negatively charged membrane that transports cations (i.e., the cation-exchange membrane). Forward bias BPMs are BPMs in which ions are transported from outer solutions towards the so-called bipolar junction.
"In forward bias, the electrolyzer is polarized in such a way that acidic cations can enter the cation-exchange membrane, and basic anions can enter the anion-exchange membrane," Toh explained. "These acidic and basic ions can then meet at the interface between the two membranes and undergo a neutralization reaction. For example, hydrogen ions and hydroxide ions meet to make water."
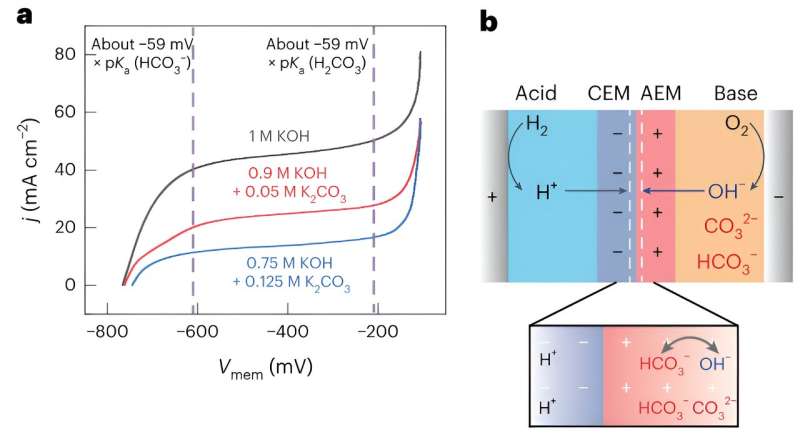
The interface between the cation-exchange and anion-exchange membranes is known to also be associated with a drop in voltage (i.e., the membrane voltage). Notably, recent experimental studies found that this membrane voltage is linked to how acidic or basic the interface is.
"The membrane voltage hence controls which acids and bases can react in the BPM," Toh said. "If you have a mixture of a weak basic anion and a strong basic anion in one of the solutions, say acetate and hydroxide, then these two anions can both enter the anion exchange membrane.
"However, depending on the membrane voltage, maybe only hydroxide, which is the more reactive ion, can react. In that case, the acetate ion would still be present in the anion exchange membrane, but end up blocking the movement of hydroxide, because it takes up space in the membrane."
The impeded movement of some ions due to other ions blocking them is known as an "ionic blockade." Ionic blockades can significantly limit the current that can pass through an electrolyzer without resulting in markedly higher voltages.
"In a sense, the way that these ions get around each other is like a sidewalk shuffle: They share the same 'path,' so to speak, and the path allows passage for only one ion at any one point, so the ions need to do a little dance and move in concert to allow current to flow," Toh said.
"For example, hydroxide and acetate exchange places so that the hydroxide can move towards the center of the BPM, and the acetate can get out of its way and move away from the center. The actual picture of how the ions move is a lot more complicated, but this sidewalk shuffle analogy is a good heuristic for starting to think about ion motion in BPMs."
In their paper, Surendranath and his colleagues outline a new model that better explains how the efficiency with which forward bias BPMs recover energy is impacted by ionic blockades. While the researchers explained their model using acetate solutions as an example, it also applies to other solutions that can block hydroxide motion, such as carbonate,
The ionic blockade phenomenon outlined by Surendranath and his colleagues directly impacts the operation of CO2 electrolyzers. In fact, increased concentrations of acetate or carbonate are known to lead to severe increases in cell overpotentials.
"First and foremost, we hope that our article helps to highlight not just the challenges, but also the promise of BPMs, and bring them to the fore of energy research," Surendranath said. "Forward bias BPM devices have already been demonstrated in many academic studies, especially as CO2 electrolyzers, but often they are not recognized as such, and so opportunities for understanding how to better optimize the energy efficiency of these devices are missed."
In the future, the model introduced by this team of researchers could inform the development of new strategies to overcome the issue of ionic blockades in BPMs, which generalize well across all forward bias BPM devices. Surendranath and his colleagues hope that this will help to further refine BPMs and improve the performance of electrolyzers, thus facilitating their widespread implementation.
"There are still aspects of how BPMs operate that remain unclear to us, we are continuing to make strides in our understanding," Surendranath added. "In particular, we are interested in the molecular details of how the interface at the center of the BPM operates and hope to be able to bring a fresh perspective to this as inorganic chemists."
More information: Wei Lun Toh et al, The role of ionic blockades in controlling the efficiency of energy recovery in forward bias bipolar membranes, Nature Energy (2023). DOI: 10.1038/s41560-023-01404-7
© 2024 Science X Network