June 19, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A perovskite for the efficient photocatalytic conversion of ethane into ethylene and hydrogen
Ethylene (C2H4) is a highly flammable compound widely used in numerous settings, including the manufacturing of packaging and plastic-based items, the chemical industry, agriculture and health care. Developing effective and sustainable methods to produce ethylene on a large scale could help to meet the rising demand for this versatile hydrocarbon.
One possible way of producing ethylene entails converting the hydrocarbon ethane (C2H6), which is abundant in natural gas, into ethylene and hydrogen. Proposed methods to do this, however, require substantial electrical power and are thus associated with a significant carbon footprint.
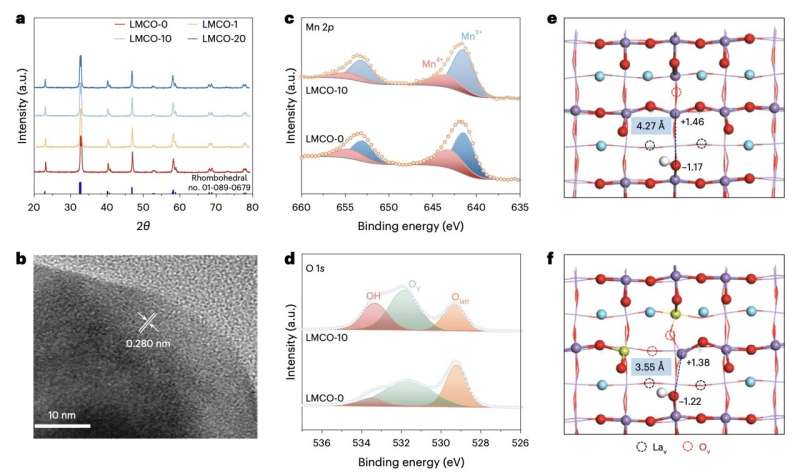
Researchers at Soochow University, University of Toronto and other institutes recently introduced a new approach to produce ethylene via the solar-powered photocatalytic dehydrogenation of ethane using the perovskite oxide LaMn1−xCuxO3. This perovskite, outlined in a paper published in Nature Energy, possesses favorable properties that make it a promising selective photocatalyst for converting ethane to ethylene and hydrogen using solar or LED light.
"Industrial-scale ethylene production occurs primarily by fossil-powered steam cracking of ethane—a high-temperature, high-energy process," Rui Song, Guanshu Zhao and their colleagues wrote in their paper. "An alternative, photochemical, pathway powered by sunlight and operating under ambient conditions could potentially mitigate some of the associated greenhouse gas emissions. We report the photocatalytic dehydrogenation of ethane to ethylene and hydrogen using LaMn1−xCuxO3."
Song, Zhao and their colleagues showed that using the perovskite oxide LaMn1−xCuxO3 as a photocatalyst, they could efficiently convert ethane into ethylene and hydrogen without the need for external heat sources. This conversion can be achieved using solar or LED lights, thus resulting in lower carbon emissions.
"This perovskite oxide possesses redox-active Lewis acid sites, comprising Mn(III) and Mn(IV), and Lewis base sites, comprising O(-II) and OH(-I), collectively dubbed surface-frustrated Lewis pairs," the team wrote. "We find that tuning the relative proportions of these sites optimizes the activity, selectivity and yield for ethane dehydrogenation."
The researchers evaluated their solar-powered ethylene production approach using a rooftop prototype device and attained remarkable ethylene production rates. In addition, they carried out comprehensive technical and economic analyses, which highlighted the sizable economic potential of their proposed solution.
"The highest ethylene production rate and ethane conversion achieved were around 1.1 mmol g−1 h−1 and 4.9%, respectively," Song, Zhao and their colleagues wrote. "We show a simple outdoor prototype to demonstrate the viability of a solar ethylene process. In addition, techno-economic analysis revealed the economic potential of an industrial-scale solar ethylene production from ethane."
To maximize the absorption of light and minimize losses, the photocatalyst and photoreactor supporting this ethane conversion process will need to be carefully engineered. Yet findings gathered so far are highly promising, suggesting that this new perovskite oxide-based photocatalyst could prove advantageous for the future large-scale production of ethylene.
In their next studies, Song, Zhao and their colleagues plan to further investigate the performance of their photocatalyst and photoreactor, specifically exploring their effects on catalytic reactions. In addition, they hope to further improve photochemical activation, light capture and light transport rates, to further boost the LaMn1−xCuxO3 perovskite's photocatalytic efficiency.
More information: Rui Song et al, Ethylene production via photocatalytic dehydrogenation of ethane using LaMn1−xCuxO3, Nature Energy (2024). DOI: 10.1038/s41560-024-01541-7
© 2024 Science X Network