This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Capturing carbon with energy-efficient sodium carbonate−nanocarbon hybrid material
Industrial emissions are one of the main sources of climate change-inducing carbon dioxide (CO2). While adopting renewable and clean energy alternatives is one option for mitigating these carbon emissions, carbon capture technology is another solution to control CO2 emissions.
In big CO2-emitting industries, such as cement, oil refineries, and thermal power plants, carbon capture technology can be easily applied to remove CO2 emissions directly at the source at a feasible cost and with low energy consumption. Different materials have been explored for CO2 capture in factories, including zeolites, metal−organic frameworks, natural minerals, alkalis, and alkali metal salts. Among them, alkali metal carbonates, such as sodium carbonate (Na2CO3), are considered effective and inexpensive materials with stable properties and easy procurement.
Theoretically, Na2CO3 has a decent CO2 capture capacity and can be easily regenerated for successive uses. However, directly applying Na2CO3 to capture CO2 causes crystal agglomeration, leading to poor efficiency and shorter longevity. This issue can be eliminated by using a carbon skeleton for Na2CO3.
Porous carbon materials with good pore connectivity provide low density, structural stability, hydrophobicity, and a large surface area that can stabilize Na2CO3. Previous studies report that Na2CO3−carbon nanocomposites have a CO2 capture capacity of 5.2 mmol/g. However, these studies do not inspect the effect of the carbonization temperatures on the overall performance of the material.
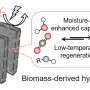
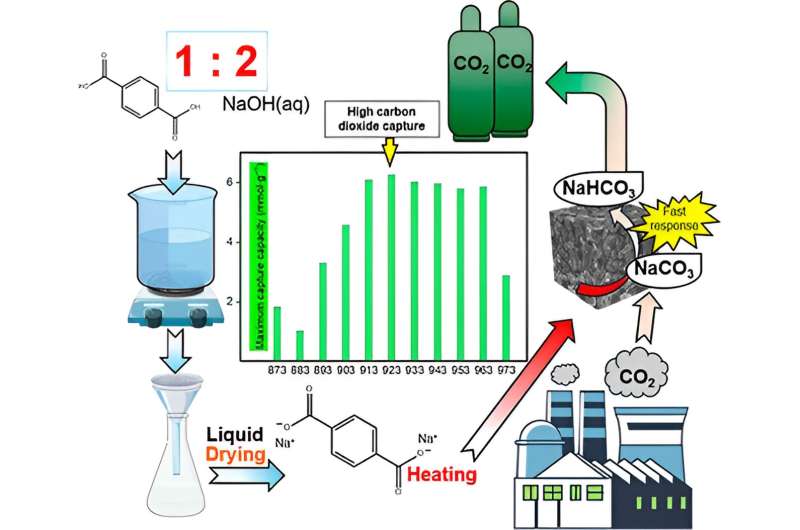
In a study published in Energy & Fuels on June 12, 2024, Professor Hirofumi Kanoh and Bo Zhang from the Graduate School of Science, Chiba University, synthesized a hybrid CO2 capture material consisting of Na2CO3 wrapped with porous nanocarbon.
They further evaluated its CO2 capture and regeneration efficiencies at different carbonization temperatures. The Na2CO3−carbon hybrids (NaCH) were derived by carbonization of disodium terephthalate at temperatures ranging from 873K to 973 K in the presence of nitrogen as a protective gas.
"Reducing CO2 emissions is an urgent issue, but research on the methods and material systems for CO2 capture are still lacking. This Na2CO3−carbon hybrid system proved promising in our initial investigations, prompting us to explore it further," states Prof. Kanoh.
The team measured the hybrid materials' CO2 capture capacity under humid conditions to mimic the conditions of factory waste exhaust gases. They found that the NaCH hybrids prepared at carbonization temperatures near 913–943 K demonstrated higher CO2 capture capacities.
Among them, NaCH-923 had the highest CO2 capture capacity of 6.25 mmol/g and a high carbon content of over 40%, which resulted in a larger surface area, enabling a more uniform distribution of Na2CO3 on the nanocarbon surface. This reduced the rate of Na2CO3 crystal agglomeration and led to faster reaction rates.
After NaCH-923 effectively captured CO2, the scientists again heated the resultant NaCH-923-CO2 in the presence of nitrogen to test its regeneration performance. They found that NaCH-923 could be regenerated and used for CO2 capture for 10 cycles, while retaining over 95% of its initial CO2 capture capacity. These results indicate that NaCH-923 exhibits good structural strength, durability, and regeneration, which makes it an excellent material for CO2 capture under humid conditions.
Further experiments on the NaCH-923-CO2 showed that the sample underwent a steep mass change at 326−373 K (around 80 °C on average). Since the temperature of the exhaust gas from thermal power plants is also typically in that range, the waste heat from factories and power plants can easily be used as a heat source for regenerating NaCH-923, thereby effectively reducing energy consumption.
These findings show that the carbonization temperature significantly influences the CO2 capture performance and carbon content of NaCH hybrids, with NaCH-923 exhibiting the best characteristics. NaCH-923, being a solid adsorbent, can efficiently capture CO2 at ambient temperature and pressure with high selectivity for CO2 and without the problem of equipment corrosion that exists with liquid adsorbents currently used in industries.
Moreover, these characteristics allow for its widespread application in various configurations, environments, and diverse industrial settings.
"By transforming Na2CO3, which already has a good CO2 capture capacity, into a nanocomposite, it became possible to improve the reaction rate and reduce the decomposition and regeneration temperature. This enables the use of factory waste heat for regeneration at around 80 °C, giving us an energy-cost efficient CO2 capture system," concludes Prof. Kanoh.
More information: Bo Zhang et al, Sodium Carbonate–Carbon Hybrid Material for Low-Energy-Consuming CO2 Capture, Energy & Fuels (2024). DOI: 10.1021/acs.energyfuels.4c01232