This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Achieving a supercapacitor through the 'molecular coating' approach

Researchers at Tohoku University have successfully increased the capacity, lifetime durability, and cost-effectiveness of a capacitor in their pursuit of a more power-efficient future. The research is published in the journal ACS Applied Materials & Interfaces.
A capacitor is a device used as part of a circuit that can store and release energy, just like a battery. What makes a capacitor different from a battery is that it takes much less time to charge. For example, your cellphone battery will power your phone instantly, but charging that battery back up to 100% when it dies is far from instantaneous.
While this makes capacitors sound like the superior choice, they have some big drawbacks that need to be overcome. First, their capacity is much smaller than batteries, so they cannot store large amounts of energy at once. Second, they can be quite expensive.
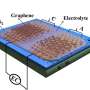
In recent years, supercapacitors (capacitors with increased capacity and performance) have been developed using nanocarbon materials, such as carbon nanotubes (CNTs), which increase the surface area and overall capacity. However, due to the expensive nature of nanocarbon materials, large-scale production using this technique is not cost-effective.
In order to tackle these specific concerns to improve the overall performance of capacitors, a research group consisting of Professor Hiroshi Yabu (Tohoku University), AZUL Energy Co., Ltd. (a venture company from Tohoku University), and the AZUL Energy x Tohoku University Bio-Inspired GX Co-Creation Center was formed.
The team succeeded in increasing the capacity of capacitors by 2.4 times (to 907 F/gAC) compared to carbon alone by "sprinkling" iron azaphthalocyanine (FeAzPc-4N), a type of blue pigment, onto activated carbon.
This method allows the molecule to adsorb at the molecular level, utilizing its redox capabilities. Additionally, the study demonstrated that 20,000 charge-discharge cycles are possible even in high-load regions of 20 A/gAC, making it possible to power LEDs.
"This increased lifespan compared to batteries may help reduce waste, as the same capacitor can be reused many more times," comments Yabu. "The components of capacitors are also significantly less toxic than batteries."
The capacitor electrode developed in this research can increase capacity to the level of supercapacitors using CNTs while utilizing commonly available and inexpensive activated carbon, making it a potential option for next-generation energy devices. The next step for the team is to make the supercapacitor even more super-powered.
More information: Kosuke Ishibashi et al, A Molecular Adsorption Concept for Increasing Energy Density of Hybrid Supercapacitors, ACS Applied Materials & Interfaces (2024). DOI: 10.1021/acsami.4c06084