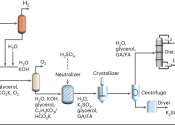
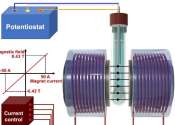
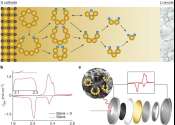
'Artificial leaf' successfully produces clean gas
A widely-used gas that is currently produced from fossil fuels can instead be made by an 'artificial leaf' that uses only sunlight, carbon dioxide and water, and which could eventually be used to develop a sustainable liquid ...
Oct 21, 2019
2
9249