Team develops stable, efficient, anode-free sodium battery
When it comes to batteries, lithium-ion are the best we have as far as energy density and convenience.
May 3, 2021
1
2527
Electronics & Semiconductors

When it comes to batteries, lithium-ion are the best we have as far as energy density and convenience.
May 3, 2021
1
2527
Engineering

Long-lasting, quick-charging batteries are essential to the expansion of the electric vehicle market, but today's lithium-ion batteries fall short of what's needed—they're too heavy, too expensive and take too long to charge.
May 12, 2021
1
137
Energy & Green Tech
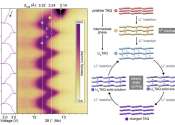
In the switch to "greener" energy sources, the demand for rechargeable lithium-ion batteries is surging. However, their cathodes typically contain cobalt—a metal whose extraction has high environmental and societal costs. ...
Jan 18, 2024
1
88
Engineering

Imagine an entire 20-storey concrete building that can store energy like a giant battery. Thanks to unique research from Chalmers University of Technology, Sweden, this vision could someday be a reality. Researchers from ...
May 18, 2021
2
306
Machine learning & AI

A team of researchers at the University of Memphis has recently developed a low-cost and portable traffic monitoring system (TMS) called DeepWiTraffic. This new system, presented in a paper pre-published on arXiv, combines ...
Energy & Green Tech

Prof. Liu Zhaoping's team at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences (CAS) has developed an electrolyte engineering strategy for lithium (Li) metal batteries ...
Jan 27, 2021
0
723
Energy & Green Tech

The rapid development of flexible and wearable electronics is giving rise to an exciting range of applications, from smart watches and flexible displays—such as smart phones, tablets, and TV—to smart fabrics, smart glass, ...
Jan 31, 2018
0
612
Energy & Green Tech

According to the International Energy Agency (IEA), global hydrogen demand is expected to reach 530 million tons in 2050, a nearly six-fold increase from 2020.
Oct 4, 2023
0
39
Electronics & Semiconductors

Carefully designed covalent organic frameworks could make supercapacitor electrodes that have a greater ability to store electric charge.
Nov 2, 2020
0
176
Energy & Green Tech

The way to boost electron transfer in grid-scale batteries is different than researchers had believed, a new study from the University of Michigan has shown. The findings are a step toward being able to store renewable energy ...
Jan 21, 2021
0
294
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ (the Greek letter rho). In some cases (for instance, in the United States oil and gas industry), density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight. Different materials usually have different densities, so density is an important concept regarding buoyancy, purity and packaging. Osmium and iridium are the densest known metal elements at standard conditions for temperature and pressure but not the densest materials.
Less dense fluids float on more dense fluids if they do not mix. This concept can be extended, with some care, to less dense solids floating on more dense fluids. If the average density (including any air below the waterline) of an object is less than water (1000 kg/m3) it will float in water and if it is more than water's it will sink in water.
In some cases density is expressed as the dimensionless quantities specific gravity (SG) or relative density (RD), in which case it is expressed in multiples of the density of some other standard material, usually water or air/gas. (For example, a specific gravity less than one means that the substance floats in water.)
The mass density of a material varies with temperature and pressure. (The variance is typically small for solids and liquids and much greater for gasses.) Increasing the pressure on an object decreases the volume of the object and therefore increase its density. Increasing the temperature of a substance (with some exceptions) decreases its density by increasing the volume of that substance. In most materials, heating the bottom of a fluid results in convection of the heat from bottom to top of the fluid due to the decrease of the density of the heated fluid. This causes it to rise relative to more dense unheated material.
The reciprocal of the density of a substance is called its specific volume, a representation commonly used in thermodynamics. Density is an intensive property in that increasing the amount of a substance does not increase its density; rather it increases its mass.
This text uses material from Wikipedia, licensed under CC BY-SA