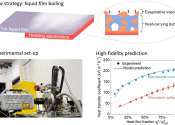
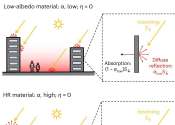
A high-fidelity model for designing efficient thermal management surfaces
In the past decade, fires from electronic devices and batteries, from small smartphones to electrical vehicles and airplanes, have repeatedly made headlines. Enhanced computational power has led to a large amount of waste ...
18 hours ago
0
1