April 25, 2016 report
How metal-organic frameworks could help realize a carbon-neutral energy cycle
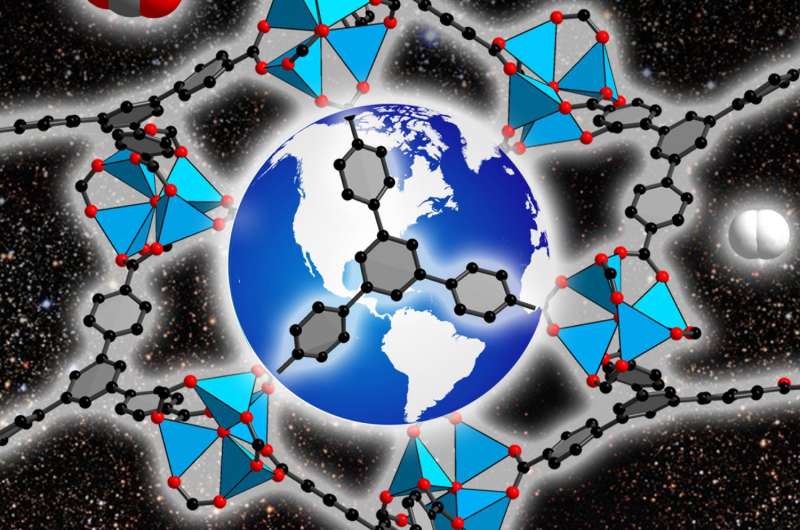
(TechXplore)—Concerns over climate change have prompted researchers to search for alternative energy sources to fossil fuels. Fossil fuels produce CO2, a greenhouse gas, but our reliance on fossil fuels will likely continue into the foreseeable future until an efficient and sustainable alternative fuel source can be realized. One option is to use metal-organic frameworks both as a short-term solution for capturing and converting CO2 and as a long-term solution for hydrogen production and storage.
Alexander Schoedel, Zhe Ji, and Omar M. Yaghi of the University of California in Berkley and Lawrence Berkley National Laboratory have written a review article outlining the current and future possibilities of using metal-organic frameworks as an intermediate and short-term solution to rising CO2 levels from burning fossil fuels and as a tool to eventually creating a carbon-neutral energy cycle. Their review appears in Nature Energy.
Metal-organic frameworks (MOFs) have drawn attention for their versatility in structural design and exceptional porosity. They are comprised of metal-oxide units that are covalently bonded to linkers. This produces a structure with interior pores whose size and shape can be tuned based on which organic and inorganic linkers are used. Importantly, gases such as H2 and CO2 can be trapped within these pores. Furthermore, many MOFs are highly stable in a broad range of temperatures and pressures.
According to Dr. Zhe Ji MOFs are attractive avenues of research for developing a carbon-neutral energy cycle because "MOFs outpace other materials in the study of these problems and are beginning to show higher and more promising performance than any other class of porous solids."
Hydrogen
A hydrogen-based fuel source is the ultimate goal because hydrogen burns cleanly and produces only water as a byproduct. However, there are practical problems involved in hydrogen storage. Whether stored as a gas or liquid, hydrogen storage still requires low temperatures under high pressure, which is expensive to maintain. Additionally, hydrogen production costs would need to be reduced by a factor of four before hydrogen can be economical competitive with fossil fuels.
If a MOF could be designed to help house hydrogen gas, this might help with the storage problem. For this to be feasible, the MOF would require a very high surface area (i.e., highly porous). Advances in this area have produced two MOFs, MOF-177 and MOF-210. Both can house a gravimetrically large amount of hydrogen (14.0 wt% and 15.0 wt%, respectively), but both require extremely low temperatures representing a current practical limitation. Other theoretical models would require a complex synthesis that would be prohibitively expensive. Research continues to look at novel MOF designs could house hydrogen gas at conditions appropriate for practical use and would be relatively inexpensive to produce.
Methane
Until hydrogen gas can be harnessed as a practical alternative for energy storage, natural gas can serve as a viable bridge between hydrogen and gasoline. Natural gas is a fossil fuel, whose main chemical component is methane (CH4), which, when burned, produces much less CO2 than gasoline does. Additionally, natural gas infrastructures and mining facilities are already in place in many countries to produce and transport natural gas. Natural gas vehicles are being developed as a feasible mode of transportation; however, larger storage amounts are needed to be energetically comparable to gasoline. This research is being addressed by the Yaghi group in collaboration with BASF.
Because the volume of natural gas needed to make the energetic equivalent of gasoline is so high, highly porous MOFs could provide a solution to natural gas storage. The Advanced Research Projects Agency-Energy of the U.S. Department of Energy (DOE) has a new program, Methane Opportunities for Vehicular Energy that has set targets for a feasible methane storage system. These targets include a gravimetric capacity of 0.5 g of methane per gram of sorbent or 700 cm3 methane per gram of sorbent at 298 K and 65 bar.
To date, MOFs are beginning to meet this target and even when they fall short of the DOE targets, their applicability is real. They can store three times the amount of natural gas compared to containers without MOFs. Some MOFs that come close to the target also provide good model systems for investigating potential MOF structures that might meet these criteria. This includes a recently reported aluminum MOF, Al-soc-MOF-1, which reached the gravimetric target of 0.5g of methane per gram of sorbent at 288K and 80 bar. However, it could not meet the DOE's target for working capacity.
Carbon dioxide
The immediate need is to somehow decrease the amount of CO2 produced as a byproduct of burning fossil fuels in order to meet the 2015 UN Climate Change Conference Goals. Two ways that MOFs can be helpful in this area is in CO2 capture and conversion.
One technology that is already in place is CO2 capture from post-combustion flue gas mixtures. While current technologies are somewhat effective, the biggest problem is selectively capturing the CO2 product quickly and efficiently.
This is where MOFs may be helpful because their pore size and chemical features can be tailored to selectively capture certain molecules. For example, Mg-MOF-74 has the highest reported CO2 uptake of 37.9 wt% at room temperature. It has a high surface area and strong open metal sites. However, Mg-MOF-74 and some of its derivatives do not necessarily work well for real-world situations such as post-combustion flue sites because these sites are typically a mixture of gases that include CO2, N2, and water.
Alternative MOFs that selectively adsorb CO2 either do so via chemical modifications that react with CO2 (i.e., chemisorption) or interact through van der Waals forces (i.e., physisorption). Another option is physically modifying the pore size to tailor it to the size of the CO2 molecule. Examples of MOFs that have a narrower pore size (~3.8 Å) have selectively absorbed CO2 in wet conditions. However, MOF selectivity is often pressure or temperature dependent. Additionally, several types of MOFs are water-sensitive, which is problematic at post-combustion flue sites.
Another option for decreasing the amount of CO2 emitted into the atmosphere is CO2 conversion. Instead of merely capturing CO2, MOFs and metallated covalent organic frameworks (COFs) can serve as a catalyst for a reaction in which CO2 is converted to a more useful compound. These reactions typically involve converting carbon dioxide into carbon monoxide.
Overall, research with MOFs is very promising for eventually achieving a carbon-neutral energy cycle. MOFs focus on the small molecules involved in both clean energy, as in the case of H2, and transitional fuel, as in CH4. In the meantime, MOFs could be used to lower the concentration of CO2 that is emitted into the environment as a result of burning fossil fuels.
More information: Alexander Schoedel et al. The role of metal–organic frameworks in a carbon-neutral energy cycle, Nature Energy (2016). DOI: 10.1038/nenergy.2016.34
Abstract
Reducing society's reliance on fossil fuels presents one of the most pressing energy and environmental challenges facing our planet. Hydrogen, methane and carbon dioxide, which are some of the smallest and simplest molecules known, may lie at the centre of solving this problem through realization of a carbon-neutral energy cycle. Potentially, this could be achieved through the deployment of hydrogen as the fuel of the long term, methane as a transitional fuel, and carbon dioxide capture and sequestration as the urgent response to ongoing climate change. Here we detail strategies and technologies developed to overcome the difficulties encountered in the capture, storage, delivery and conversion of these gas molecules. In particular, we focus on metal–organic frameworks in which metal oxide 'hubs' are linked with organic 'struts' to make materials of ultrahigh porosity, which provide a basis for addressing this challenge through materials design on the molecular level.
© 2016 TechXplore