Controlling dendrites reveals secret to rechargeable lithium electrode

Imagine your cellphone being as thin as a piece of paper and not in need of near-constant charging.
That's possible with the manipulation of a battery's dendrites, says Jian Xie, an Indiana University-Purdue University Indianapolis professor of mechanical engineering and a research member of the IUPUI Integrated Nanosystems Development Institute.
In a paper recently published in Nature Energy, Xie and a team of authors—including School of Engineering and Technology postdoctoral research associates Yadong Liu and Le Xin, postgraduate student Qi Liu, and Ph.D. student Fan Yang—detail how they have solved a decades-old problem with lithium metal electrodes in batteries.
For battery manufacturers, how to make a rechargeable lithium metal electrode has proved frustratingly elusive. Such an electrode would make batteries 10 times more powerful than those currently used in a number of applications.
The fault lies in the dendrites.
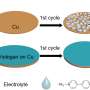
Dendrites are crystals that grow inside lithium metal electrodes, in a style similar to trees. As they grow in every direction, they eventually span the entire electrode and short-circuit the battery, making it no longer rechargeable.
Engineers have tried to prevent dendrites from forming at all, but that goes against the metal's nature. Instead, Xie and his team allow the dendrites to form but change their growth direction so they grow in a densely packed layer that doesn't adversely affect the battery. The end result is revolutionary, allowing for safe recharging and high capacity.
"We designed the working principle based on electroplating, and the experiment turned out to work exactly as designed—a rarity in my 35-year research career," Xie said. "The possibilities for this application are numerous, and we feel the work can be further improved in the years to come."
Lithium metal electrodes have the highest theoretical specific capacity of energy density, at 3,863 mAh/g (milliAmpere-hour/grams). By comparison, an everyday graphite/hard carbon electrode is only 330 mAh/g.
The expected applications for such a breakthrough battery could include microelectronics, medical devices, electric vehicles, drones, military uses and, yes, portable electronics such as cellphones.
More information: Yadong Liu et al. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction, Nature Energy (2017). DOI: 10.1038/nenergy.2017.83