Chemists develop polymer cathodes for ultrafast batteries
In the face of the surging demand for lithium-ion batteries and limited lithium reserves, scientists are searching for alternatives to the lithium technology. Russian researchers from Skoltech, D. Mendeleev University, and the Institute of Problems of Chemical Physics of RAS have synthesized and tested new polymer-based cathode materials for lithium dual-ion batteries. The tests showed that the new cathodes withstand up to 25,000 operating cycles and charge in a matter of seconds, thus outperforming lithium-ion batteries. The cathodes can also be used to produce less expensive potassium dual-ion batteries. The research was published in the journal Energy Technology.
The amount of electricity consumed worldwide grows by the year, and so does the demand for energy storage solutions, since many devices often operate in autonomous mode. Lithium-ion batteries can generate enormous power while showing fairly high discharge and charge rates and storage capacity per unit mass, making them a popular storage device in electronics, electric transport, and global power grids. For instance, Australia is launching a series of large-scale lithium-ion battery storage projects to manage excess solar and wind energy.
If lithium-ion batteries continue to be produced in growing quantities, the world could run out of lithium reserves. With Congo producing 60% of cobalt for lithium-ion battery cathodes, cobalt prices may skyrocket. The same goes for lithium, as the water consumption in lithium mining poses a great challenge for the environment. Therefore, researchers are looking for new energy storage devices relying on more accessible materials while using the same operating principle as lithium-ion batteries.
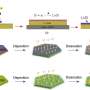
The team used a promising post-lithium dual-ion technology based on the electrochemical processes involving the electrolyte's anions and cations to attain a manifold increase in the charging rate as compared to lithium-ion batteries. Another plus is that the cathode prototypes were made of polymeric aromatic amines which can be synthesized from various organic compounds.
"Our previous research addressed polymer cathodes for ultra-fast high-capacity batteries that can be charged and discharged in a few seconds, but we wanted more," says Filipp A. Obrezkov, a Skoltech Ph.D. student and the first author of the paper. "We used various alternatives, including linear polymers, in which each monomeric unit bonds with two neighbors only. In this study, we went on to study new branched polymers where each unit bonds with at least three other units. Together they form large mesh structures that ensure faster kinetics of the electrode processes. Electrodes made of these materials display even higher charge and discharge rates."
A standard lithium-ion cell is filled with lithium-containing electrolyte and divided into the anode and the cathode by a separator. In a charged battery, the majority of lithium atoms are incorporated in the anode's crystal structure. As the battery discharges, lithium atoms move from the anode to the cathode through the separator. The Russian team studied the dual-ion batteries in which the electrochemical processes involved the electrolyte's cations (i.e. lithium cations) and anions that get in and out of the anode and cathode material's structures, respectively.
Another novel feature is that, in some experiments, the scientists used potassium electrolytes instead of expensive lithium ones to obtain potassium dual-ion batteries.
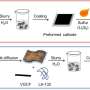
The team synthesized two novel copolymers of dihydrophenazine with diphenylamine (PDPAPZ) and phenothiazine (PPTZPZ) which they used to produce cathodes. As anodes, they used metallic lithium and potassium. Since the key features of these battery prototypes called half-cells are driven by the cathode, the scientists assemble them in order to quickly assess the capabilities of new cathode materials.
While PPTZPZ half-cells showed average performance, PDPAPZ turned out to be more efficient: lithium half-cells with PDPAPZ were fairly quick to charge and discharge, while displaying good stability and retaining up to a third of their capacity even after 25,000 operating cycles. If a regular phone battery were as stable, it could be charged and discharged daily for 70 years. PDPAPZ potassium half-cells exhibited a high energy density of 398 Wh/kg. For comparison, the value for common lithium cells is 200-250 Wh/kg, the anode and electrolyte weights included. Thus the Russian team demonstrated that polymer cathode materials can be used to create efficient lithium and potassium dual-ion batteries.
More information: Filipp A. Obrezkov et al. Dihydrophenazine‐Based Copolymers as Promising Cathode Materials for Dual‐Ion Batteries, Energy Technology (2020). DOI: 10.1002/ente.202000772