December 9, 2021 feature
Study explores the degradation mechanisms in Cu2O photoelectrodes for CO2 reduction
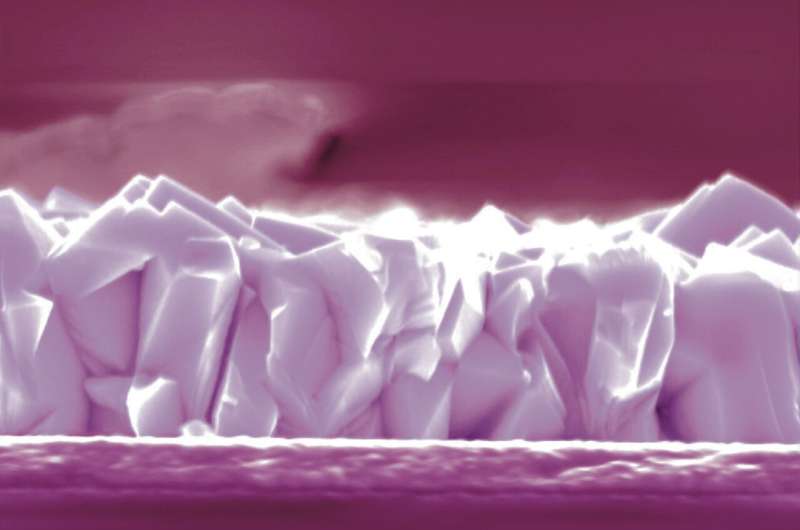
The efficient and large-scale conversion of carbon dioxide into fuels or other valuable substances could help to address the current energy crisis and mitigate the effects of global warming. One of the most effective approaches for converting CO2 into other products, using sunlight and at ambient temperature and pressure, is a process known as photoelectrochemical (PEC) CO2 reduction.
While this process has achieved promising results so far, it requires the use of semiconductor materials that are prone to degradation. Understanding the chemical changes underpinning this degradation could ultimately help to devise strategies to mitigate it and reduce its adverse effects.
Researchers at the Lawrence Berkeley National Laboratory have recently carried out a study investigating the mechanisms underpinning the degradation of cuprous oxide (Cu2O). In their paper, published in Nature Energy, they also propose a strategy that could help to mitigate this material's degradation during PEC CO2 reduction processes.
"The PEC CO2 reduction process is also known as artificial photosynthesis because it mimics nature's energy cycle," Francesca Maria Toma, one of the researchers who carried out the study, told TechXplore. "Semiconductor materials (e.g., Cu2O) can work as photoelectrodes and are key to this process. However, existing photoelectrodes usually suffer from catastrophic degradation under operating conditions."

So far, the degradation of photoelectrodes has prevented the development of PEC systems that could realistically substitute existing energy conversion and storage technologies. While some past studies introduced techniques to protect semiconductors, the mechanisms underpinning their degradation are still poorly understood.
"In our paper, we determine the degradation mechanism of Cu2O, as a model photocathode, under operation, identifying key factors that govern the degradation process," Toma explained. "Consequently, we provide a rational design of a protection scheme for this material that ensures sustained and selective light-driven CO2 reduction to ethylene."
As part of their study, Toma and her colleagues conducted a series of experiments aimed at better understanding the origin of the corrosion that takes place in Cu2O photoelectrodes. To do this, they gathered extensive chemical, structural and functional data using numerous different tools.
"For example, we utilized inductively coupled plasma mass spectrometry (ICP-MS), X-ray photoelectron spectroscopy (XPS), scanning electron microscope (SEM), high-resolution transmission electron microscopy (HRTEM) and photoelectrochemical (PEC) characterizations, as well as operando ambient-pressure X-ray photoelectron spectroscopy (APXPS) to understand the degradation pathways of Cu2O under illumination in different electrolytes relevant to hydrogen production and CO2 reduction," Toma said. "This latter technique, applied at the Advanced Light Source, was instrumental to complete the puzzle, revealing the special role of hydroxide ions in the corrosion process."
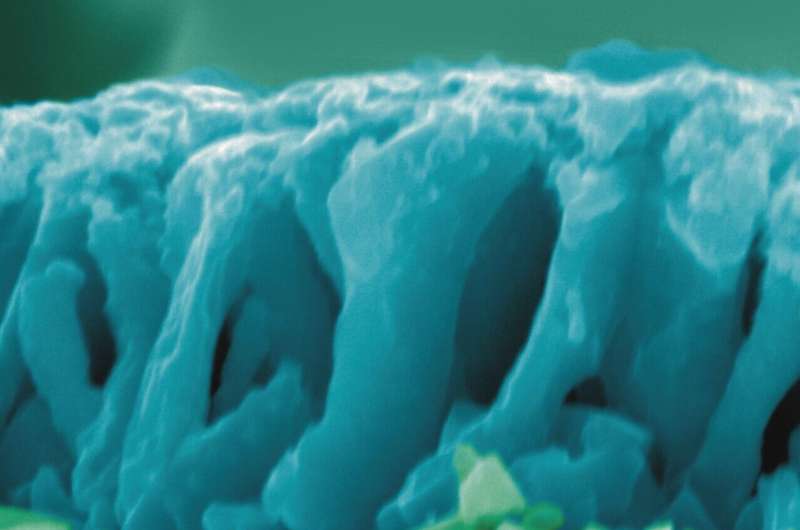
The experiments conducted by the researchers yielded several interesting results. Most notably, the team observed that under illumination Cu2O concurrently undergoes reduction by photogenerated electrons and oxidation by holes in the material, at electrolyte-dependent degradation rates.
These findings inspired Toma and colleagues to devise a new technique for protecting photoelectrodes. This technique entails the use of a silver catalyst to accelerate the transfer of photogenerated electrons and a Z-scheme heterojunction to extract the holes in the material.
-

Francesca Maria Toma and Guiji Liu, two of the researchers who carried out the study. Credit: Thor Swift/Berkeley Lab. © The Regents of the University of California, Lawrence Berkeley National Laboratory. -

Guiji Liu working on the project. Credit: Thor Swift/Berkeley Lab. © The Regents of the University of California, Lawrence Berkeley National Laboratory. -
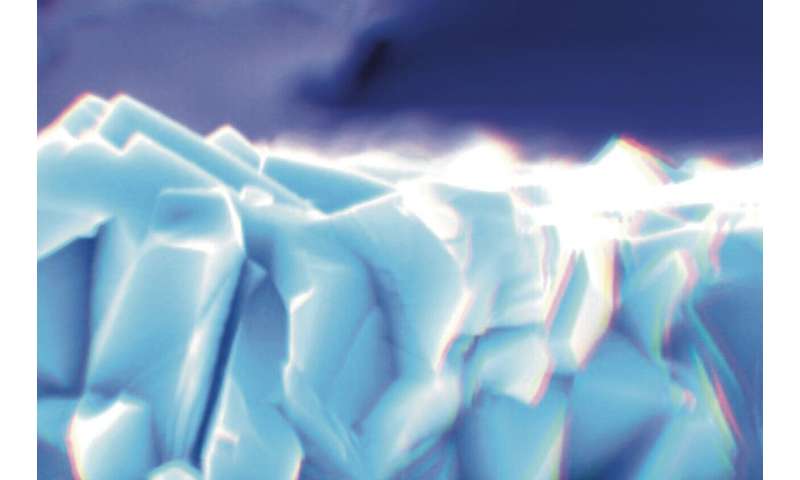
Colored scanning electron microscopy pictures of the samples used by the researchers. X-section of pristine sample. Credit: Adriana Prakhova (Ella Maru Studio Inc.).
"Our work highlights the importance of surrounding electrolytes in defining the kinetic transformation pathway, which is currently overlooked in the literature," Toma said. "Moreover, it suggests that understanding materials transformations under operating conditions is foundational to rational design of solar-driven devices to incorporate photoelectrodes in new architectures and environments."
In the future, the findings gathered by this team of researchers could facilitate the widespread adoption of PEC CO2 reduction processes, by helping to mitigate the degradation of photoelectrodes. Meanwhile, Toma and her colleagues plan to conduct further studies assessing new energy solutions and technologies.
"We will continue to develop new solar fuel devices for liquid fuels production by this rational approach—understanding how materials and device transform while they are functioning can enable preventive repair and prolonged activity," Toma said. "We want to keep a close eye to sustainability and circularity to make sure that our materials and approaches can be relevant in the energy quest."
More information: Guiji Liu et al, Investigation and mitigation of degradation mechanisms in Cu2O photoelectrodes for CO2 reduction to ethylene, Nature Energy (2021). DOI: 10.1038/s41560-021-00927-1
© 2021 Science X Network















