March 22, 2022 feature
New ferrocenium-based anion-exchange membranes for fuel cells
Anion exchange membranes (AEMs) are semipermeable fuel cell components that can conduct anions but reject cations and gases. This enables the partition of substances that could chemically react with one another, thus allowing the cells to operate properly.
A team of researchers at Tianjin University in China have recently developed new types of AEMs that are based on a newly designed ferrocenium material. Their membranes, presented in a paper published in Nature Energy, were found to achieve highly promising results in terms of power output, durability, and ohmic resistance.
"As the oriented mixed-valence ferrocenium material developed in our study is entirely new for the AEM field, we encountered many difficulties and frustrations along the way," Michael D. Guiver, one of the researchers who carried out the study, told TechXplore. "We spent a long research period and much effort, both experimentally and theoretically, to achieve these good outcomes. The whole process from initial conceptualization to final publication was convoluted, but fortunately successful."
The recent study by Guiver and his colleagues builds on two of their previous works, where they introduced new proton exchange membrane materials that were magnetically responsive and had conducting capabilities. The ultimate goal of their previous studies was to orient conductive channel structures under a magnetic field, which would in turn extend alignment strategies to the field of AEMs.
"While it builds on our past efforts, our study is also an independent pioneering work, as it employs different material systems in a different field," Guiver said. "Our primary objectives were to build through-plane oriented highly conductive channels in AEMs, to promote anion transport because of the more aligned and direct conductive channels, and concurrently realize robust membrane durability in fuel cell application. We were happy to achieve both."
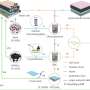
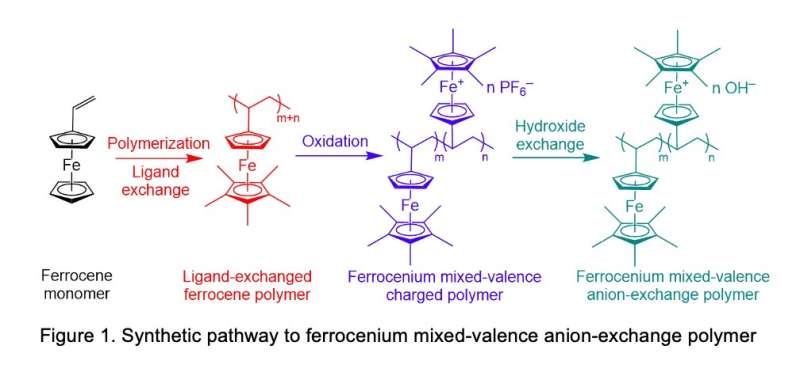
To ensure that the polymer they developed would be an effective AEM and that it could be influenced by magnetic fields, the researchers initially started looking at a material system that contained iron, namely ferrocene. Some past studies had explored similar materials (i.e., metallocenes), such as cobaltocene, and found that AEMs based on these materials achieved a good stability and high conductivity. However, past findings found that metallocenes could not align under magnetic fields, as they are not paramagnetic.
"An alternative possibility was to use ferrocene, which when made into a polymer, and oxidized, might be paramagnetic and also bear the positive charge needed to function as an AEM," Guiver explained. "However, the use of iron molecules in fuel cell membranes has been largely avoided, because in some forms, they accelerate degradation."
In their previous work, Guiver and his colleagues had introduced ferrocyanide into proton exchange membranes. When they did this, they found that some materials containing iron could improve the stability of fuel cells. Inspired by these results, they now decided to explore the potential of using polymers containing ferrocene as AEMs, which had so far never been assessed.
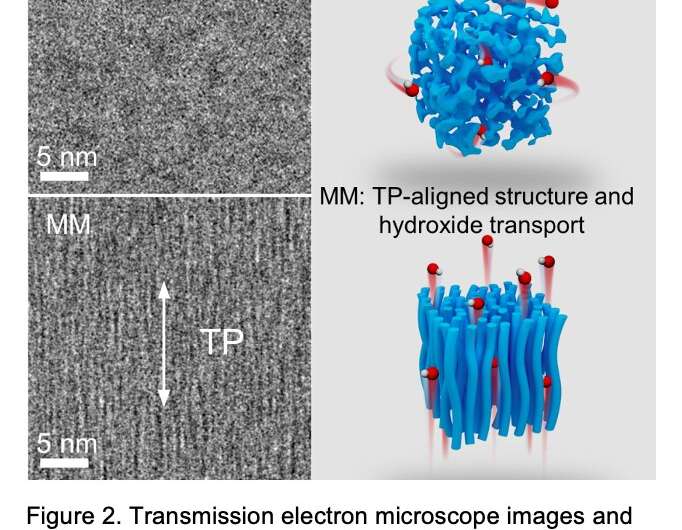
"Our study focused on both high performance and robust durability of AEMs," Guiver said. "The ferrocenium material with both magnetic responsiveness and conducting ability cast under magnetic field realized conducting channel alignment, and the mixed-valence ferrocenium-ferrocene state induced by magnetic field led to very good durability."
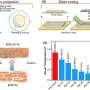
The new ferrocenium-based AEMs introduced by Guiver and his colleagues have a magnetic field-oriented and conductive structure. In addition, they have a robust durability, with a 3.9% voltage loss and a 2.2% high-frequency resistance increase over 500 h at 500 mA cm−2, 120 °C and 40% relative humidity.
"For anion transport, most anion-exchange membranes show isotropic conductivity (i.e., the same amount of conductivity in the in-plane and through-plane direction)," Guiver said. "Ideally, since anions transport between electrodes across the membrane (i.e., through-plane direction), shorter and more direct channels should be a big benefit to make the cell perform well."
To enhance membrane durability, conventional AEMs typically include positively charged materials containing nitrogen. While the use of these materials can be effective in making cells more durable, often they result in issues with stability, due to chemical reactions that cause cell degradation. As the membranes created by Guiver and his colleagues combine a through-plane-oriented conductive structure with an alternative anion conductor based on ferrocenium, they could help to circumvent these key drawbacks of conventional AEMs.
"We were the first to use ferrocenium cations, allowing through-plane oriented conductive structure, and the mixed-valence state, affording extraordinary durability," Guiver explained. "These findings can be distilled into the following outcomes: high membrane hydroxide conductivity in the through-plane direction; no appreciable conductivity loss over an alkali stability test period of 4,320 hours (180 days); simultaneous improvements in both conductivity and stability; lowest membrane thickness-normalized high-frequency resistance; and operation and considerable durability at 120 °C and 40% relative humidity."
In the future, the AEMs introduced by this team of researchers could be used to develop stable, durable, and highly performing fuel cells that can operate for a long time at elevated temperatures. The initial results gathered by Guiver and his colleagues suggest that their membranes could promote reaction kinetics on electrodes, as well as the migration of catalyst poisoning by CO and membrane carbonation from CO2 by high-temperature desorption/purging. In addition, they could help to simplify cell cooling and humidification systems.
"Our membranes could also facilitate the alleviation of water flooding in the anode, enhance water diffusivity through the membrane to overcome water management issues, and improve the efficiency of waste heat caused by an increased temperature difference between cell stack and coolant," Guiver added. "We are now developing alternative material systems with both external field responsiveness and conducting ability. We aim to achieve stronger orientation under weaker magnetic field, which would decrease cost and allow commercial scalability."
More information: Xin Liu et al, Magnetic-field-oriented mixed-valence-stabilized ferrocenium anion-exchange membranes for fuel cells, Nature Energy (2022). DOI: 10.1038/s41560-022-00978-y
Xin Liu et al, Magnetic field alignment of stable proton-conducting channels in an electrolyte membrane, Nature Communications (2019). DOI: 10.1038/s41467-019-08622-2
Xin Liu et al, Oriented proton-conductive nano-sponge-facilitated polymer electrolyte membranes, Energy & Environmental Science (2019). DOI: 10.1039/C9EE03301G
Xiangwen Zhang et al, A paradigm shift for a new class of proton exchange membranes with ferrocyanide proton-conducting groups providing enhanced oxidative stability, Journal of Membrane Science (2020). DOI: 10.1016/j.memsci.2020.118536
Xin Liu et al, Durability enhancement of proton exchange membrane fuel cells by ferrocyanide or ferricyanide additives, Journal of Membrane Science (2021). DOI: 10.1016/j.memsci.2021.119282
© 2022 Science X Network