This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists develop new electrolytes for low-temperature lithium metal batteries
Electric vehicles, large-scale energy storage, polar research and deep space exploration all have placed higher demands on the energy density and low-temperature performance of energy storage batteries. In recent years, lithium metal batteries with a high specific capacity of lithium metal anode have become one of the most promising high energy density batteries.
However, in the carbonate electrolytes, solvent molecules interact strongly with Li+, which consequently hinders the migration of Li+ and the stability of the lithium metal interface. This limitation restricts the application of lithium metal batteries in low-temperature environments.
A research team led by Prof. Li Feng from the Institute of Metal Research of the Chinese Academy of Sciences has proposed a new electrolyte design strategy to regulate the energy of oxygen bonding in the solvent to achieve exceptional performance of lithium metal batteries even under low-temperature conditions.
This work was published as a supplementary cover article in Journal of the American Chemical Society.
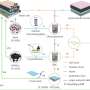
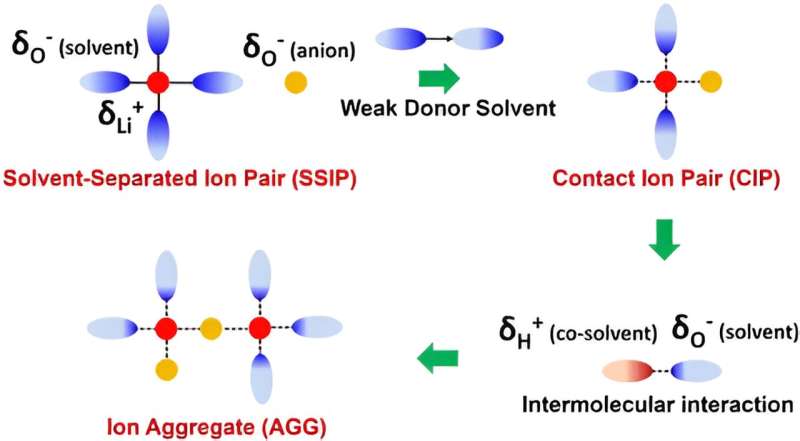
In this study, the energy of oxygen bonding in the solvent was found to be closely associated with ionic coordination and interfacial transport. The effect of different oxygen bonds, such as sulfone (S=O), ester (C=O), and ether (C–O) on the structure and temperature adaptability of the electrolyte was elucidated.
Through extensive screening, tetrahydrofuran-based ether solvents with weak oxygen bonding were selected. On this basis, the interaction between Li+ and solvents was further attenuated by hydrogen bonding between fluorinated solvent and ether solvent molecules.
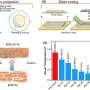
This strategy significantly accelerates the desolvation process of Li+ and reduces the side effects of solvents on interfacial transport and stability. The lithium metal batteries exhibited a high reversibility with 100% capacity retention after 150 cycles at room temperature, -20℃ and -40℃.
This is one of the most stable low-temperature lithium metal batteries reported in the literature. The practical Ah-level battery exhibited excellent performance with this new electrolyte, and this strategy provides a novel approach to the development of electrolytes for low-temperature batteries.
More information: Nan Piao et al, Designing Temperature-Insensitive Solvated Electrolytes for Low-Temperature Lithium Metal Batteries, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c01735