July 7, 2022 report
Block copolymer electrolyte used to make stable sodium metal batteries
A team of researchers affiliated with a host of institutions in Australia, the U.S. and China has found that a perfluoropolyether-based electrolyte can be used to make stable sodium metal batteries. They have published their results in Nature Materials.
Lithium-based batteries currently dominate the battery market, but that is expected to change as the demand for batteries grows and the supply of lithium shrinks. But to make that change, scientists must find a suitable alternative. One possibility is sodium-based batteries, mostly because sodium is so plentiful and easily obtained. Unfortunately, sodium batteries have a history of igniting or exploding. But scientists believe such outcomes can be averted by finding the right electrolyte. In this new effort, the researchers have found an electrolyte that worked well when tested for some applications.
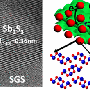
The researchers did not create the electrolyte from scratch (another team did that); they simply repurposed it for use in batteries. The electrolyte was perfluoropolyether-based, which meant it was a block copolymer, which is a type of molecule that has two major compounds that exist in alternating forms in its structure. In this case, one of the molecule types was a carbon/sulfur compound and the other was a hydrocarbon with the majority of its hydrogen atoms replaced with fluorine. The researchers noted that the material switched from glass to plastic at slightly elevated temperatures, the kind that would be present in a battery.
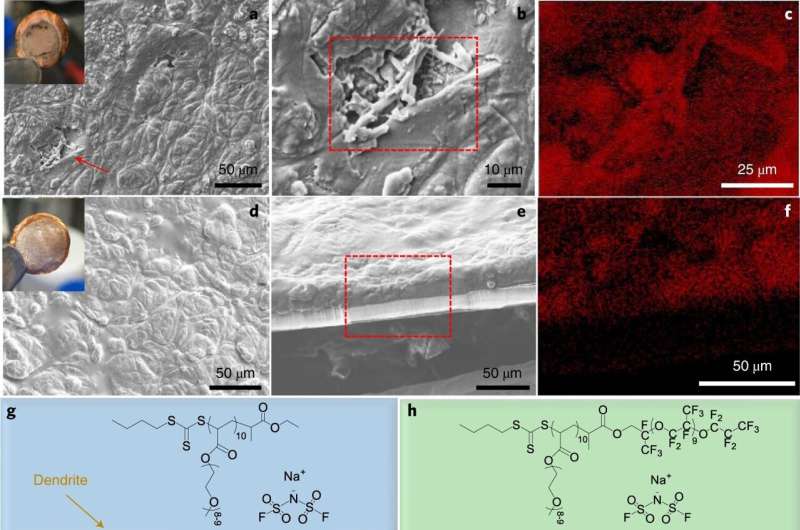
To test the material as a suitable electrolyte in a battery, they built two similar prototypes—both of them had one sodium metal electrode. They differed in the makeup of the other electrode. In one of the prototypes, the other electrode was made using a sodium-vanadium phosphate while the other was made using sodium iron phosphate.
Both approaches worked, in the sense that both were able to hold a charge and both were able to do so after 900 cycles—each was capable of holding 97% of initial capacity. They also found that both were much more stable than other sodium-based batteries, suggesting that despite their high weight, they might find use in applications such as storage devices for alternative energy farms.
More information: Xiaoen Wang et al, Ultra-stable all-solid-state sodium metal batteries enabled by perfluoropolyether-based electrolytes, Nature Materials (2022). DOI: 10.1038/s41563-022-01296-0
© 2022 Science X Network