A cost-effective way to transform water into fuel
Finding sustainable sources of renewable energy will help combat climate change and offer consumers access to reliable sources of fuel.
Researchers from the Nano and Molecular Systems Research Unit (NANOMO) at the University of Oulu in Finland, have developed a cost-effective way to transform water into fuel. Their new nickel-based catalyst (a substance that accelerates the speed of a chemical reaction) uses sunlight to split water into oxygen and hydrogen, allowing them to harness the hydrogen as a source of energy. The team's findings were recently published in Applied Energy.
"Solar water splitting directly converts solar energy into hydrogen fuel," Harishchandra Singh, Adjunct Professor, said. "Since a renewable non-carbon source like solar is used, the hydrogen produced would also be a renewable source of energy in the true sense."
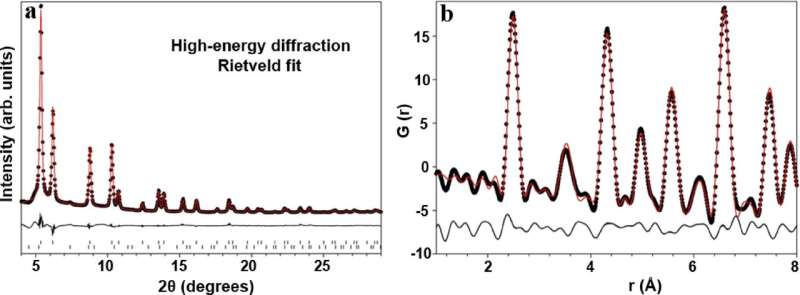
With the help of the Brockhouse beamline at the Canadian Light Source (CLS) located at the University of Saskatchewan (USask), the team was able to analyze the materials they used for their catalyst—allowing them to understand why their design was so effective.
"The beamline gives us access to very intense beams of high-energy X-rays which allow us to see details on the surface of these materials that are hard to see with other techniques," said Graham King, a scientist on the Brockhouse beamline.
Precious metals are often used in hydrogen fuel cells, making the production of hydrogen-based energy expensive. Instead, Singh and his team used nickel, which is considerably more affordable.
Singh has been using synchrotron technology for years and relies on facilities like the CLS for his research on structural, building, and energy materials that can support a circular economy.
"Because interactions within the material are happening at the nanoscale, this research would be very hard without the synchrotron," Singh said.
More information: Parisa Talebi et al, Unveiling the role of carbonate in nickel-based plasmonic core@shell hybrid nanostructure for photocatalytic water splitting, Applied Energy (2022). DOI: 10.1016/j.apenergy.2022.119461