March 20, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A photocatalyst that can produce hydrogen peroxide from oxygen and water
Hydrogen peroxide (H2O2) is a colorless liquid compound with strong oxidizing properties that can have numerous industrial and medical applications. This compound is generally synthesized through the anthraquinone process, through which the compound anthraquinone combined with palladium-based materials catalyzes the oxidation of dihydrogen (H2) to H2O2 with O2 from air.
H2O2 could also theoretically be synthesized through photocatalysis, a chemical reaction that entails the absorption of light by reacting elements through the addition of catalysts. However, this has been difficult to realize reliably using existing catalysts, which may respond weakly to sunlight, require sacrificial reagents or are not sufficiently active during the reaction.
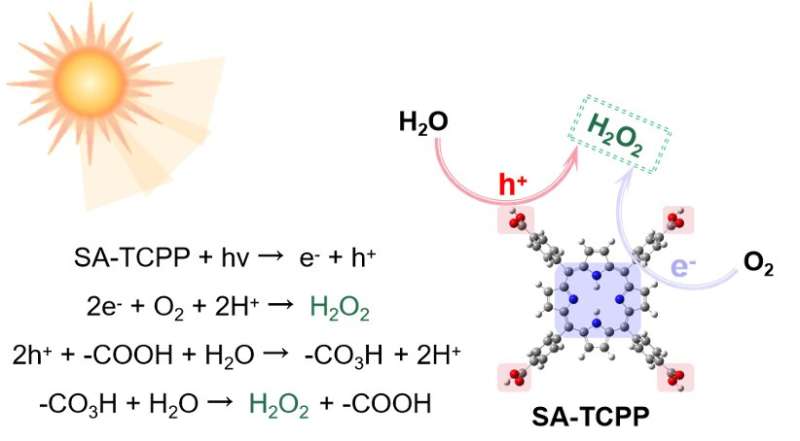
Researchers at Jiangnan University and Tsinghua University recently introduced a new photocatalyst that could be used to reliably produce H2O2 from only H2O and O2 through photocatalysis. This photocatalyst, presented in a paper in Nature Energy, is based on self-assembled tetrakis(4-carboxyphenyl)porphyrin, a compound sometimes used to analyze or remove metals.
"Photocatalytic H2O2 production only requires H2O, O2 and sunlight, which is considered as a promising alternative to the industrial anthraquinone method," Chengsi Pan, one of the researchers who carried out the study, told Tech Xplore. "However, due to a high Gibbs free energy of 117 kJ mol−1, most photocatalytic systems require sacrificial reagents (e.g., EtOH, IPA) to generate considerable H2O2. Our group has been working since 2018 to develop a photocatalyst that can continuously produce H2O2 without sacrificial reagents."
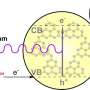
In one of their previous works, Pan and his colleagues had tested the potential of bismuth phosphate (BiPO4) as a photocatalyst for producing H2O2. While their results were promising, they found that BiPO4 only responded to UV light, which limited its use and prevented solar energy-based applications. In their new study, they thus set out to evaluate another photocatalyst, which was based on the narrow bandgap tetrakis(4-carboxyphenyl)porphyrin supramolecule (TCPP).
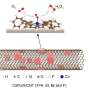
"We employed a self-assembled tetrakis(4-carboxyphenyl)porphyrin nanosheet (SA-TCPP) to achieve the desired activity," Pan explained. "This material was prepared by dissolving commercial bulk TCPP in NaOH and subsequently adding HCl. For the H2O2 generation experiments, a certain amount of SA-TCPP was added to a glass bottle containing only water and O2 bubbling."
To evaluate the activity of their photocatalyst, Pan and his colleagues irradiated the glass bottle with the solution at an air mass 1.5 spectra (AM 1.5G) using a solar simulator, and then heated it at an elevated temperatures of 353 K. In initial tests, the SA-TCPP photocatalyst exhibited a near-infrared wavelength quantum efficiency of 1.1% at 940 nm and a high solar-to-chemical conversion efficiency of ca. 1.2%.
"Encouraged by these results, we evaluated the photocatalysts in a lab-made flow reactor, which resulted in a H2O2 accumulation concentration of ca. 1.1 wt%, a level close to the commercial one (3 wt%), which is the first example that produces a commercial level of H2O2 via the photocatalytic method," Pan said.
"We then also discovered that SA-TCPP could perform H2O2 generation through a dual pathway of O2 reduction and H2O oxidation, a unique characteristic compared to most previously reported photocatalysts. Notably, we observed that the introduction of -COOH can help the H2O oxidation pathway by converting to -CO3H groups, which subsequently undergo thermal hydrolysis."
The results of initial tests conducted by this team of researchers are highly promising, highlighting the value of their proposed photocatalyst for the large-scale production of H2O2 using solar light. In the future, their work could inform the design of other organic photocatalysts that can achieve even higher solar efficiencies, enabling the non-sacrificial generation of H2O2.
"We now plan to explore two potential areas for future work: the integration of our photocatalyst with photothermal conversion materials, and the redesign of the reactor to achieve increased production amount beyond bench scale," Pan added.
"By combining our photocatalyst with photothermal conversion materials, we can raise its local temperature only through light irradiation, reducing the need for external heat sources as reported in our current paper. Additionally, we aim to develop a scalable prototype device for H2O2 production with SA-TCPP using only air, H2O, and sunlight by optimizing the reactor design and reaction parameters."
More information: Yaning Zhang et al, H2O2 generation from O2 and H2O on a near-infrared absorbing porphyrin supramolecular photocatalyst, Nature Energy (2023). DOI: 10.1038/s41560-023-01218-7
© 2023 Science X Network