This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New recipes for better solar fuel production

Just as a superb meal requires the right ingredients prepared expertly, the production of better green fuel alternatives requires combining the right materials and methods. Recently, a team of researchers from China and the U.K. have found new ways to optimize the recipe for the production of solar fuels. Their findings have been published in two articles, one in the journal Applied Surface Science, and the other in Optical Materials.
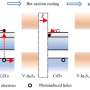
Hydrogen is a zero-emission energy source that can be produced from water using solar energy and offers great potential in helping to mitigate the climate crisis. The process of producing hydrogen from water is called "water splitting" because it breaks water into its two elements, hydrogen and oxygen. Water splitting requires a semiconductor photocatalyst, a substance or compound that absorbs sunlight and then uses its energy for the splitting process.
However, semiconductor photocatalysts for water splitting vary in their efficiency. By using novel combinations of methods and materials to create new types of photocatalysts, the researchers have improved the efficiency of hydrogen production.
Dr. Graham Dawson, who led the studies at Xi'an Jiaotong-Liverpool University, explains, "By adding materials such as gold or boron nitrate to our photocatalysts using particular mixing methods, we can increase the amount of light that is absorbed.
"The more light that is absorbed, the more suitable energy there is for water splitting, and so hydrogen production is increased," he says.
Finding the perfect recipe
Modifying the materials commonly used as photocatalysts helps to overcome their limitations, says the first author of the article in Applied Surface Science, Yanan Zhao. One of the most widely used materials is titanium dioxide. "Titanium dioxide can harness energy directly from the sun with negligible pollution and shows great potential in the development of solar-related technologies," she says.
"However, it can only be activated by UV light, which accounts for only 7% of sunlight. It cannot absorb the energy of visible light," explains Zhao, who received her master's degree in chemistry from XJTLU and was awarded a full scholarship to pursue her Ph.D. at the University of North Dakota.
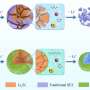
The researchers found that adding boron nitride to a form of titanium dioxide produced a photocatalyst that can absorb the energy of more wavelengths than UV light. Boron nitride, a compound of boron and nitrogen, has good electrical conductivity and can withstand temperatures of up to 2,000°C.
Zhao explains the process: "To prepare the composite photocatalytic material, we combined boron nitride with titanate nanotubes, which are tube-like structures with dimensions measured in nanometers—one nanometer is one-billionth of a meter.
"By optimizing the ratio of boron nitride to titanate nanotubes and using chemical processes to combine the compounds, we produced a very stable composite photocatalyst. It can absorb light from a wider range of wavelengths and produce more hydrogen compared to traditional physical mixing methods."
A gold rush
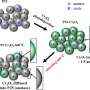
In the second study, published in Optical Materials, Dr. Dawson's team found an additional option for improving photocatalytic efficiency in water splitting. They covered the surfaces of specific types of photocatalytic structures with a specific size of gold nanoparticles thereby increasing the amount of light they could absorb.
Shiqi Zhao, this study's first author, explains, "The structure of the photocatalytic material used is very important. In this study, we used two forms of photocatalytic nanostructures—nanosheets and nanotubes. We coated them with differently sized gold particles to see which combination produced the highest amount of hydrogen from water.
"Our results showed that the nanosheets modified with small, uniform gold particles had the best photocatalytic performance out of the materials we tested. These gold-coated nanostructures showed approximately 36 times more photocatalytic hydrogen production performance than unmodified nanotubes," he continues.
"This provides a new understanding of how semiconductor photocatalytic materials can be modified with gold nanoparticles and has valuable applications in the fields of photocatalytic hydrogen production, solar cells and optical sensors."
More information: Yanan Zhao et al, Enhanced photocatalytic hydrogen production by the formation of TiNT-BN bonds, Applied Surface Science (2023). DOI: 10.1016/j.apsusc.2023.157005
Shiqi Zhao et al, Enhanced photocatalytic activity through anchoring and size effects of Au nanoparticles on niobate nanotubes and nanosheets for water splitting, Optical Materials (2023). DOI: 10.1016/j.optmat.2023.113753