July 20, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new self-cleaning salt molecule to improve the performance of lithium metal batteries
Lithium (Li) metal batteries, batteries that contain a metallic Li-based anode, are currently used to power a wide range of electronic devices. Most of these batteries contain carbonate-based electrolytes, which are unable to passivate the corrosion of the lithium anode, causing the substantial growth of dendrites and limiting a cell's cycling life.
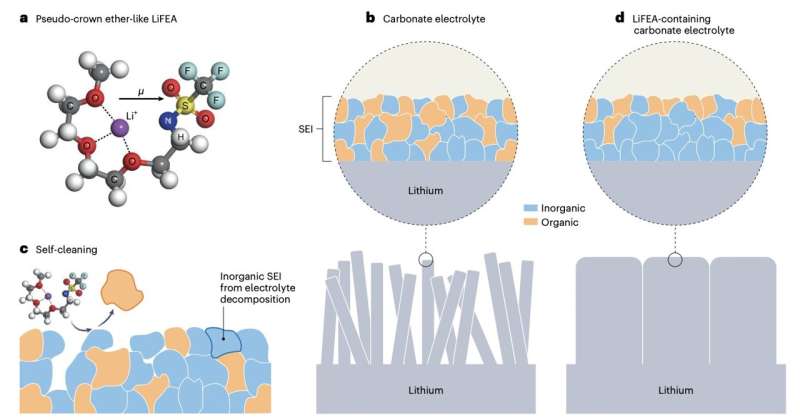
Researchers at Tsinghua University and other institutes in China recently designed lithium 1,1,1-trifluoro-N-[2-[2-(2-methoxyethoxy)ethoxy)]ethyl] methanesulfonamide (LiFEA), a new asymmetric Li salt with an ether-like molecular geometry that could improve the performance of Li metal batteries. This salt, introduced in a paper published in Nature Energy, enables a self-cleaning mechanism that cleanses the solid-electrolyte interphase (SEI), a protective layer that forms on the surface of lithium-based anodes as the electrolyte is decomposed.
"Lithium metal batteries has been recognized as the next generation batteries, for their high energy density," Kai Liu, one of the researchers who carried out the study, told Tech Xplore. "However, the organic-rich solid electrolyte interphase that forms on the lithium metal electrode in conventional carbonate electrolytes is unable to passivate the electrode surface against lithium corrosion, and thus the life, power and safety of the battery is severely limited. We designed a new lithium salt molecule with a novel self-cleaning ability to remove organic components from the interphase, which leads to high power and long life performance with carbonate electrolytes."
LiFEA, the new lithium salt created by Liu and his colleagues, has a characteristic pseudo-crown, ether-like, and folded molecular geometry. This unique geometry promotes the formation of an SEI rich of inorganic materials, which is highly compatible with lithium metal anodes.
The salt was synthesized by substituting a specific component of commonly used lithium salts with an ethylene glycol-based chain. In initial tests, the researchers found that their adding their salt to electrolytes facilitated the dissolution of initially formed SEIs, speeding it up threefold.
Spectroscopic measurements showed that the salt can slow down the corrosion of the anodes and the growth of dendrites, by prompting the SEI to "clean itself." The team found that this can significantly improve the performance of lithium metal batteries, improving both their power density and cycling stability.
"The new lithium salt we designed can self-fold to form unique secondary structures, which brings the new self-cleaning mechanism of the battery interfaces and greatly improve battery performance," Liu said.
The researchers so far introduced their new Li salt in battery cells and found that they performed remarkably well. Specifically, pouch cells of 310 Wh kg−1 achieved a power density of ~410 W kg−1 at a discharging current density of 6.59 mA cm−2.
Moreover, after 100 cycles under fast-cycling conditions (charging: 1.46 mA cm−2, discharging: 3.66 mA cm−2), these cells retained 81% of their capacity. In the future, this recent work could contribute to the fabrication of fast-cycling and high-energy Li metal batteries for a wide range of applications, including electric vehicles (EVs).
"We believe our work will increase the energy density as well as the power density of the batteries," Liu added. "In other words, an EV may charge faster, accelerate faster and with a longer driving distance in the future. We will now focus on improving the molecular structure and further improving its performance on industrial large format cells."
More information: Yingchun Xia et al, Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries, Nature Energy (2023). DOI: 10.1038/s41560-023-01282-z
© 2023 Science X Network