This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers design a new way to capture and recycle carbon dioxide from industrial emissions

Carbon capture is a promising method to help slow climate change. With this approach, carbon dioxide (CO2) is trapped before it escapes into the atmosphere, but the process requires a large amount of energy and equipment. Now, researchers reporting in ACS Central Science have designed a capture system using an electrochemical cell that can easily grab and release CO2. The device operates at room temperature and requires less energy than conventional, amine-based carbon-capture systems.
Many industries are turning to electrification to help curb carbon emissions, but this technique isn't feasible for all sectors. For example, CO2 is a natural byproduct of cement manufacture, and thus a major contributor to emissions on its own.
Excess gas can be trapped with carbon-capture technologies, which typically rely on amines to help "scrub" the pollutant by chemically bonding to it. But this also requires lots of energy, heat and industrial equipment—which can burn even more fossil fuels in the process.
Carbon capture could itself be electrified by using electrochemical cells, and these devices could be powered by renewable energy sources. So, Fang-Yu Kuo, Sung Eun Jerng and Betar Gallant wanted to develop an electrochemical cell that could easily and reversibly trap CO2 with minimal energy input.
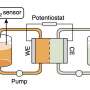
The team first developed an electrochemical cell that could both catch and release emitted carbon by "swinging" positively charged cations across a liquid amine dissolved in dimethyl sulfoxide. When the cell was discharged, a strong Lewis cation interacted with the carbamic acid, releasing CO2 and forming the carbamate amine. When the process was reversed and the cell charged, the cation was removed, and the cell could capture CO2 and reform the carbamic acid in the process.
The researchers optimized the ion-swinging process with a combination of potassium and zinc ions. In a prototype cell, they used these two ions as the basis for the cell's cathode and anode. This cell required less energy than other, heat-based cells and was competitive with other electrochemical cells in initial experiments. Additionally, they tested the device's long-term stability and found that nearly 95% of its original capacity was maintained after several cycles of charging and discharging, demonstrating that the system was feasible.
The researchers say that this work shows that an electrochemical alternative is possible and could help make continuous CO2 capture-release technologies more practical for industrial applications.
More information: Fang-Yu Kuo et al, Dual Salt Cation-Swing Process for Electrochemical CO2 Separation, ACS Central Science (2023). DOI: 10.1021/acscentsci.3c00692