August 15, 2023 dialog
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
written by researcher(s)
proofread
How the material of the battery electrode affects its performance and lifespan
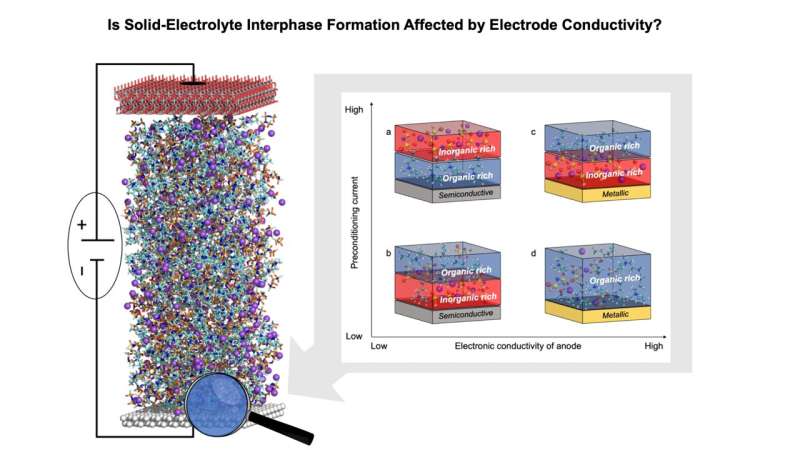
Batteries are devices that store and release energy by moving charged particles called ions between two materials called electrodes. The electrodes are separated by a liquid or gel called an electrolyte, which contains ions and other molecules. When a battery is used, a thin layer of molecules forms on the surface of each electrode, called the solid-electrolyte interphase (SEI).
Lithium-ion batteries (LIBs) and sodium-ion batteries (SIBs) suffer from deficient reversibility of redox processes at the electrode/electrolyte interface, which is associated with the formation of mechanically unstable and reactive SEIs. The stable inorganic rich SEIs can insulate electron transfer, allowing only certain ions to diffuse through, therefore supporting reversible cycling beyond the electrolyte's electrochemical limit.
While electrochemical cells utilizing inorganic molten salt electrolytes at elevated temperatures (> 100°C) exhibit stable cycling performance, everyday applications rely on battery electrolytes comprising both metal salts and organic solvents. This mixture triggers competitive reactions at the charged interface, causing ongoing electrolyte consumption and uneven metal deposition, i.e., dendrite formation in the
case of metal electrodes, resulting in battery failure and sometimes safety concerns.
One of the most practically scalable ways to optimize SEI chemistry and morphology for reversible charge transport is co-selection of electrolyte chemistry and formation protocol (i.e., initial cycling conditions, with specific current/voltage conditions). Simultaneously, the significance of the electrode material in this progression has been notably underemphasized, despite its inherent influence on the preliminary phases of SEI formation.
To bridge this information void, researchers from Deakin and Monash Universities (Melbourne, Australia) examined the effects of physicochemical properties of the electrode on the mechanism of the SEI formation with ionic liquids and carbonate-based sodium electrolytes. The work is published in the journal Energy & Environmental Science.
Using a combination of experimental and theoretical tools, we demonstrated that the structure of the electrolyte–electrode interface and the properties of the solid-electrolyte interphase are substantially affected by the polarizability of the electrode (its dielectric nature), and we explained these phenomena in the context of the ability of charged electrodes to adsorb electrolyte species (see figure above).
Specifically, nonmetallic electrodes with weak van der Waals forces encounter electrostatic repulsion, preventing the accumulation of highly polar solvents or ions carrying the same charge as the charge of the electrode. This affects the concentration of Na–anion complexes with an organic solvent near the charged electrode. Consequently, the interphase chemistries formed vary based on the applied charging conditions, leading to the development of different interphase chemistries—either solvent-derived or anion-derived.
With this new scientific discovery and knowledge about ion-solvation structure in given electrolytes, we can be more rational in the design of smart cycling protocols for rechargeable batteries.
This research could help design better batteries for various applications, such as electric vehicles, renewable energy storage, or portable devices. It could also help develop new ways to use electrochemical systems for other purposes, such as making chemicals (electrocatalysis) or recovering metals from waste.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information: Dmitrii A. Rakov et al, The impact of electrode conductivity on electrolyte interfacial structuring and its implications on the Na0/+ electrochemical performance, Energy & Environmental Science (2023). DOI: 10.1039/D3EE00864A
Dr Dmitrii A. Rakov is a Postdoctoral Research Fellow at the School of Chemical Engineering at the University of Adelaide. He received his PhD degree from Deakin University. Before joining the University of Adelaide, Dr Rakov worked as a Postdoctoral Research Fellow at the Australian Institute of Bioengineering and Nanotechnology in the University of Queensland, where he now holds an Adjunct Fellow position at Professor Chengzhong Yu’s group. Dr Rakov also was an Impossible Without You Fellow at the Commonwealth Scientific and Industrial Research Organization (CSIRO), an intern at NASA Ames Research Center, and a visiting scientist at the University of Tennessee Knoxville and Oak Ridge National Laboratory. Dr Rakov's research interests include the molecular understanding of energy storage systems and methods to optimize their performance, in particular, electrolyte, interface, and interphase.