This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Green hydrogen could reach economic viability through the co-production of valuable chemicals
It already works: there are several approaches to using solar energy to split water and produce hydrogen. Unfortunately, this "green" hydrogen has so far been more expensive than "gray" hydrogen from natural gas.
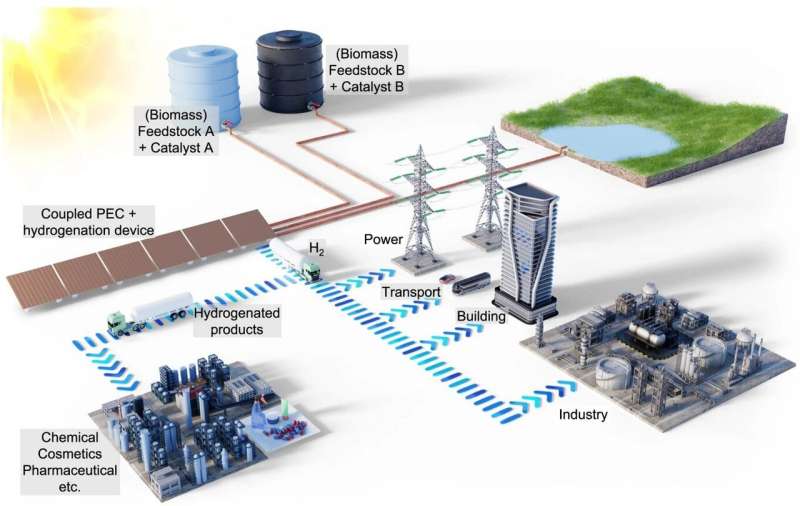
A study by the Helmholtz-Zentrum Berlin (HZB) and the Technical University of Berlin now shows how green hydrogen from sunlight can become profitable: part of the hydrogen is used to upgrade raw biomass-derived chemicals into high-value chemicals for industry. This co-production concept is very flexible; the same plant can be used to produce different by-products as required.
We need to move away from fossil fuels as soon as possible in order to limit global warming. In the energy system of the future, green hydrogen will therefore play an important role in energy storage and as a renewable feedstock for the production of chemicals and materials for a wide range of applications.
At present, hydrogen is mainly produced from fossil natural gas (gray hydrogen). Green hydrogen, on the other hand, is produced via electrolysis of water using renewable energy. One promising approach is to use photoelectrochemical (PEC) devices to produce hydrogen using solar energy. However, hydrogen from PEC plants is much more expensive than hydrogen from (fossil) methane.
Full control over reactions
A team led by Fatwa Abdi (at HZB until mid-2023, now at City University in Hong Kong) and Reinhard Schomäcker (UniSysCat, TU Berlin) has now investigated how the balance changes when some of the hydrogen produced in a PEC device reacts with itaconic acid (IA) to form methylsuccinic acid (MSA), all within the same device.
The starting material, itaconic acid, comes from biomass and is fed in. Methylsuccinic acid is a high priced compound and needed by the chemical and pharmaceutical industries.
In the study, the team describes how to control the chemical reactions in the PEC device by varying the process parameters and the concentration of the homogenous rhodium-based catalyst, which is water-soluble and already active at room temperature. In this way, different proportions of hydrogen could be used for the hydrogenation of itaconic acid, selectively increasing or decreasing the production of methylsuccinic acid.
Plant becomes profitable from 11 percent hydrogen for MSA
With a realistic overall PEC plant efficiency of 10 percent and taking into account primary costs as well as operation, maintenance and decommissioning, pure hydrogen production remains too expensive compared to production from fossil gas. This is true even if the lifetime of the PEC plant is assumed to be 40 years.
This balance changes if the PEC reaction is coupled with the hydrogenation process. Even if only 11 percent of the hydrogen produced is converted to MSA, the cost of hydrogen drops to 1.5 € per kilogram, which is already at the same level as for hydrogen from methane steam reforming. And this is true even for a PEC plant lifetime of only 5 years.
As the market price of MSA is significantly higher than that of hydrogen, more MSA increases the profitability. In the experiment, between 11 and up to 60 percent of the hydrogen could be selectively used to produce MSA.
In addition, a previous study has shown that co-production of MSA also reduces the so-called energy payback time, i.e. the time it takes for the plant to recover the energy that its production has consumed.
The PEC plant can also be used to produce other co-products by simply changing the feedstock and the (soluble) catalyst: for example, acetone could be hydrogenated to isopropanol. "This is another major advantage of our concept of co-production. We have found a promising way to make solar hydrogen production economically viable," says Fatwa Abdi.
The work is published in the journal Nature Communications.
More information: Keisuke Obata et al, Solar-driven upgrading of biomass by coupled hydrogenation using in situ (photo)electrochemically generated H2, Nature Communications (2023). DOI: 10.1038/s41467-023-41742-4

















