This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Diminishing ether-oxygen content of electrolytes enables temperature-immune lithium metal batteries
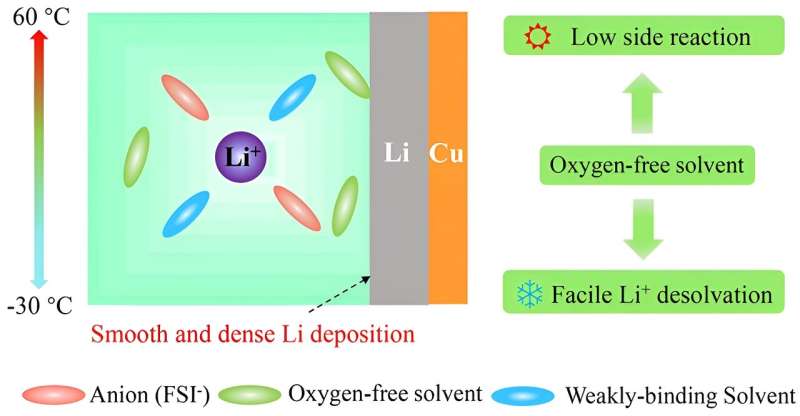
Oxygen-free n-Hexane (HEX), the most stable solvent against Li-metal, was successfully introduced into the standard concentration electrolyte to constitute an electrolyte for temperature-immune lithium-metal batteries.
Since lithium metal has the lowest electrode potential and largest specific capacity, thus employing lithium metal can push the energy density of Li-ion batteries to its limits. However, the high reactivity of lithium metal and the serious Li dendrite growth seriously restrict the development of Li-metal anodes.
Furthermore, Li-metal anode is highly temperature-sensitive, exhibiting aggravated side reactions between Li metal anodes and the electrolyte as temperatures rise and exacerbating Li dendrite growth as temperatures fall. It has failed to achieve high Coulombic efficiency (CE) and uniform Li deposition for Li metal anodes at extreme temperatures.
In a study published in the journal Science China Chemistry, researchers first proposed an oxygen-free solvent (alkane) non-reactive against lithium-metal to constitute an electrolyte for temperature-immune lithium-metal batteries. It was discovered that the introduction of oxygen-free HEX reduces the side reactions between electrolyte and Li-metal to the greatest extent and greatly promotes Li+ desolvation, leading to ultra-high Li Coulombic efficiencies (99.59% at 25℃, 99.30% at 60℃ and 98.75% at –30 ℃) and dendrite-free structure at a wide temperature range (from –30 to 60 °C).
Furthermore, their electrolyte enables the energy density of the SPAN (3.8-mAh cm–2)||Li (60-μm) pouch-cells to increase from conventional 221 to 278 Wh kg–1 under the given E/S=3.2 μL mg–1, and also maintain 248 and 320 Wh kg–1 energy density at –30 and 60 °C, respectively.
The research was led by Prof. Liumin Suo (Institute of Physics, Chinese Academy of Sciences).
More information: Tao Liu et al, Diminishing ether-oxygen content of electrolytes enables temperature-immune lithium metal batteries, Science China Chemistry (2023). DOI: 10.1007/s11426-023-1705-9