This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
New material developed for better supercapacitor applications
Supercapacitors, also known as ultracapacitors or electric double-layer capacitors (EDLCs), are advanced energy storage devices with unique characteristics. Unlike traditional batteries, supercapacitors store energy through the electrostatic separation of charges at the interface between an electrolyte and a high-surface-area electrode. This mechanism allows for rapid energy storage and release, enabling supercapacitors to deliver high power bursts and exhibit exceptional cycle life.
Supercapacitors play a pivotal role in the realm of renewable energy and environmental conservation. In the context of renewable energy, supercapacitors serve as crucial components for energy storage and delivery systems. Their ability to rapidly store and release energy makes them well-suited for smoothing out intermittent energy sources, such as solar and wind power, ensuring a consistent and reliable energy supply.
In the environmental conservation domain, supercapacitors excel as sustainable alternatives to traditional energy storage devices. Their long cycle life, fast charging/discharging capabilities, and reduced environmental impact make them environmentally friendly choices. Additionally, their application in electric vehicles and hybrid systems fosters the transition towards cleaner transportation, aligning with global efforts to reduce carbon emissions and combat climate change.
Overall, supercapacitors contribute significantly to the advancement of sustainable energy solutions and environmentally conscious practices.
Now, oxygen vacancies engineering is widely acknowledged as a potent strategy for augmenting the electrochemical performance of metal oxides in the realm of supercapacitors.
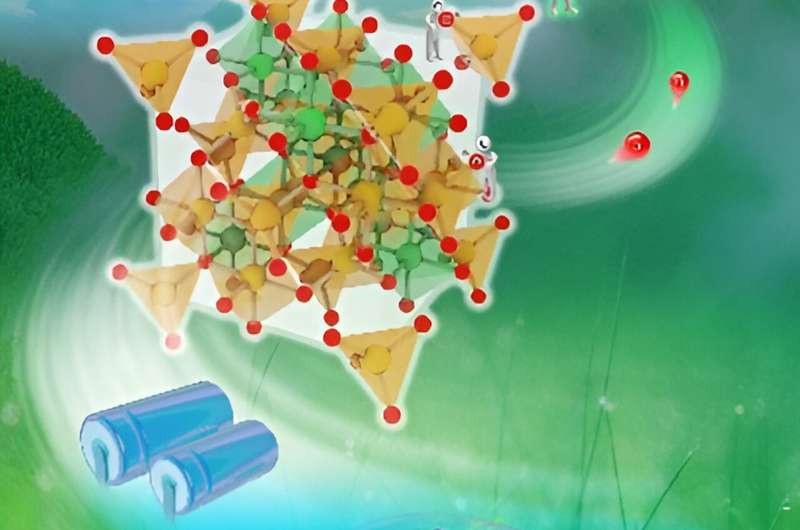
In recent research by Prof. Jianqiang Bi's team, NiFe2O4−δ, characterized by a profusion of oxygen vacancies, was successfully synthesized through a subsequent heat treatment process within an activated carbon bed, building upon the foundation of the hydrothermal-synthesized NiFe2O4.
The meticulous treatment yielded the NiFe2O4−δ, which exhibited superior conductivity and a remarkable 3.7-fold increase in capacitance compared to its NiFe2O4 counterpart.
This observed enhancement in electrochemical properties underscores the pivotal role played by oxygen vacancies in optimizing the performance of metal oxides.
The results of their study strongly support the notion that the deliberate introduction of oxygen vacancies holds substantial promise for advancing the electrochemical properties of metal oxides, thereby positioning them as promising materials for supercapacitor electrodes. Their work was published in the journal Frontiers of Chemical Science and Engineering.
This newfound understanding opens avenues for potential applications in the field of energy storage, showcasing the significant impact of oxygen vacancy engineering on the development of high-performance supercapacitors.
Prof. Jianqiang Bi's research team also includes Xicheng Gao, Linjie Meng, Lulin Xie and Chen Liu from Shandong University, China.
More information: Xicheng Gao et al, Activated carbon induced oxygen vacancies-engineered nickel ferrite with enhanced conductivity for supercapacitor application, Frontiers of Chemical Science and Engineering (2023). DOI: 10.1007/s11705-023-2352-6