March 12, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The realization of aqueous flow batteries with mild pH decoupling
Technologies that can store the energy produced by photovoltaics and wind turbines could play a key role in the decarbonization of the energy sector. The operation of both solar cells and wind turbines relies on suitable weather conditions, and grid-scale energy storage solutions could help to store the energy produced when the sun is out and the wind is blowing so that it can be used later.
Some of the most promising among these storage solutions are so-called aqueous redox flow batteries (ARFBs), which are designed to store energy in chemical solutions. These batteries have various advantages, including their safety, long lifetimes, remarkable power capacities, and low fabrication costs.
Despite these considerable advantages, fabricating reliable ARFBs with long operation lifetimes can be challenging, as the performance of these batteries heavily relies on the balance between its two sides, which contain a positively charged and a negatively charged electrolyte, respectively. Competing water-splitting reactions inside ARFB cells can compromise their Coulombic efficiency, adversely impacting the balance between these two sides and reducing the batteries' lifespan.
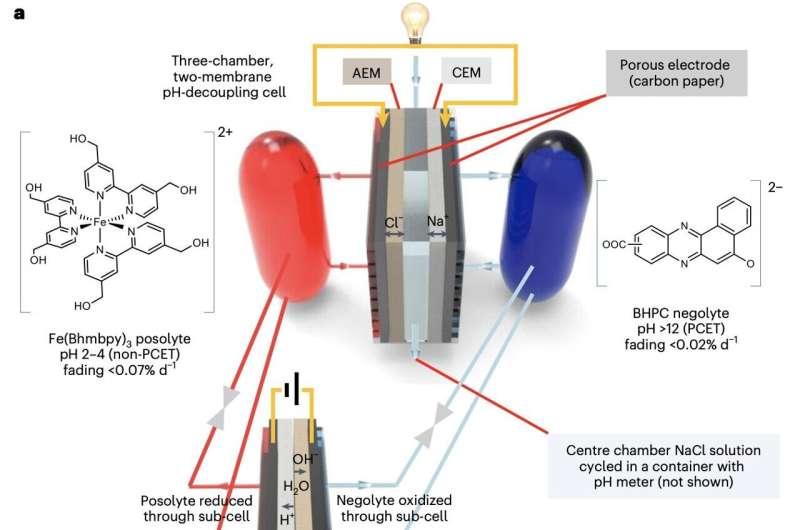
Researchers at Harvard University have recently introduced a strategy that could help to mitigate this effect, via the realization of mild pH-decoupling aqueous flow batteries. This strategy, described in Nature Energy, entails the use of mildly acidic and mildly alkaline electrolytes, to mitigate crossover between chemical solutions and thus prevent previously reported reductions in efficiency.
"Establishing a pH difference between the two electrolytes (pH decoupling) of an ARFB enables cell voltages exceeding the 1.23 V thermodynamic water-splitting window, but acid–base crossover penalizes efficiency and lifetime," Dawei Xi, Abdulrahman M. Alfaraidi and their colleagues wrote in their paper. "We employ mildly acidic and mildly alkaline electrolytes to mitigate crossover, achieving high round-trip energy efficiency with open circuit voltage >1.7 V."
The ARFB created by the researchers contains a negatively charged electrolyte with a pH of ~13, and a positively charged electrolyte with a pH of ~3. The team found that their design significantly reduced the rate of crossover between electrolytes to less than 0.3 nmol s-1 cm-2, achieving an open-circuit energy efficiency of above 1.7 V and a long operation lifetime.
"We implemented an acid–base regeneration system to periodically restore electrolytes to their initial pH values," Xi, Alfaraidi and their colleagues wrote. "The combined system exhibited capacity fade rate 85% and approximately 99% Coulombic efficiency during stable operation for over a week. Cost analysis shows that the tolerance of acid–base crossover could be increased if the pH-decoupling ARFB achieved a higher voltage output and lower resistance."
This mild pH-decoupling-based strategy employed by the researchers could soon help to improve the performance, lifetime, rate capability and energy efficiency of ARFBs. This could in turn have important implications for the future storage of energy generated from renewable energy sources, while also potentially helping to enhance grid-scale, remote power and electric vehicle charging systems.
More information: Dawei Xi et al, Mild pH-decoupling aqueous flow battery with practical pH recovery, Nature Energy (2024). DOI: 10.1038/s41560-024-01474-1
© 2024 Science X Network