Lufthansa to add environmental charge to fares
German airline giant Lufthansa said Tuesday it would add an environmental charge of up to 72 euros ($77) to fares in Europe to cover the cost of increasing EU climate regulations.
Jun 25, 2024
0
9
Business

German airline giant Lufthansa said Tuesday it would add an environmental charge of up to 72 euros ($77) to fares in Europe to cover the cost of increasing EU climate regulations.
Jun 25, 2024
0
9
Energy & Green Tech

For the millions of fans who tune into every race, Formula One (F1) is more than just a sport—it's the apex of aerodynamics, skill and strategy.
Jun 24, 2024
0
11
Engineering

A new device can measure carbon dioxide captured in concrete more simply and in a third of the time of current methods. Researchers at the University of Tokyo worked with engineers in industry to create the boxlike device ...
Jun 24, 2024
0
9
Energy & Green Tech
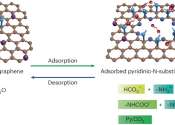
Scientists at EPFL have developed advanced atom-thin graphene membranes with pyridinic-nitrogen at pore edges, showing unprecedented performance in CO2 capture. It marks a significant stride toward more efficient carbon capture ...
Jun 24, 2024
0
68
Energy & Green Tech

In the face of the dual challenges of climate change and energy security, conducting in-depth research on energy and carbon emission (E&C) systems has become crucial for nations to address environmental issues and promote ...
Jun 20, 2024
0
3
Energy & Green Tech
Switching from gas-powered cars to electric vehicles is one way to reduce carbon emissions, but building the lithium-ion batteries that power those EVs can be an energy-intensive and polluting process itself. Now researchers ...
Jun 20, 2024
0
48
Energy & Green Tech

Green hydrogen holds many promises: it can serve as a "battery" for energy storage, it can be used in the chemical industry, and its only emission will be water vapor. But, unfortunately, green hydrogen is not yet widely ...
Jun 18, 2024
0
6
Energy & Green Tech
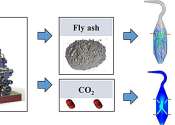
In advancing sustainable waste management and CO2 sequestration, researchers have crafted reactors that mineralize carbon dioxide with fly ash particles. This avant-garde technique is set to offer a sustainable and lasting ...
Jun 14, 2024
0
60
Engineering

This year has already seen massive heat waves around the globe, with cities in Mexico, India, Pakistan and Oman hitting temperatures near or past 50 degrees Celsius (122 degrees Fahrenheit).
Jun 13, 2024
0
26
Engineering

Thermoelectric technology, which enables the direct conversion of heat into electricity, has emerged as a promising alternative energy source. Notably, this technology can efficiently convert body heat into electrical energy, ...
Jun 12, 2024
0
18
Carbon (pronounced /ˈkɑrbən/) is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. There are three naturally occurring isotopes, with 12C and 13C being stable, while 14C is radioactive, decaying with a half-life of about 5730 years. Carbon is one of the few elements known since antiquity. The name "carbon" comes from Latin language carbo, coal, and, in some Romance and Slavic languages, the word carbon can refer both to the element and to coal.
There are several allotropes of carbon of which the best known are graphite, diamond, and amorphous carbon. The physical properties of carbon vary widely with the allotropic form. For example, diamond is highly transparent, while graphite is opaque and black. Diamond is among the hardest materials known, while graphite is soft enough to form a streak on paper (hence its name, from the Greek word "to write"). Diamond has a very low electrical conductivity, while graphite is a very good conductor. Under normal conditions, diamond has the highest thermal conductivity of all known materials. All the allotropic forms are solids under normal conditions but graphite is the most thermodynamically stable.
All forms of carbon are highly stable, requiring high temperature to react even with oxygen. The most common oxidation state of carbon in inorganic compounds is +4, while +2 is found in carbon monoxide and other transition metal carbonyl complexes. The largest sources of inorganic carbon are limestones, dolomites and carbon dioxide, but significant quantities occur in organic deposits of coal, peat, oil and methane clathrates. Carbon forms more compounds than any other element, with almost ten million pure organic compounds described to date, which in turn are a tiny fraction of such compounds that are theoretically possible under standard conditions.
Carbon is one of the least abundant elements in the Earth's crust, but the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. It is present in all known lifeforms, and in the human body carbon is the second most abundant element by mass (about 18.5%) after oxygen. This abundance, together with the unique diversity of organic compounds and their unusual polymer-forming ability at the temperatures commonly encountered on Earth, make this element the chemical basis of all known life.
This text uses material from Wikipedia, licensed under CC BY-SA