New system retrofits diesel engines to run on 90% hydrogen
Engineers from UNSW Sydney have successfully converted a diesel engine to run as a hydrogen-diesel hybrid engine—reducing CO2 emissions by more than 85% in the process.
Oct 7, 2022
7
17192
Energy & Green Tech

Engineers from UNSW Sydney have successfully converted a diesel engine to run as a hydrogen-diesel hybrid engine—reducing CO2 emissions by more than 85% in the process.
Oct 7, 2022
7
17192
Energy & Green Tech

Amid the fierce competition throughout the globe to develop hydrogen mobility technologies to achieve carbon neutrality, a new technology for a 2-liter class hydrogen-fueled engine (a passenger car hydrogen engine) capable ...
Sep 7, 2023
0
662
Energy & Green Tech

Hydrogen can be produced by electrolysis of water, ideally with solar cells or wind power providing the electrical energy required. This "green" hydrogen is expected to play an important role in the energy system of the future. ...
Mar 20, 2023
0
1234
Robotics

A team of Harvard University researchers with expertise in 3D printing, mechanical engineering, and microfluidics has demonstrated the first autonomous, untethered, entirely soft robot. This small, 3D-printed robot—nicknamed ...
Aug 24, 2016
1
9766
Energy & Green Tech

What does it take to produce green hydrogen more efficiently and cheaply? Apparently, small ruthenium particles and a solar-powered system for water electrolysis. This is the solution proposed by a joint team involving the ...
Dec 13, 2023
5
279
Energy & Green Tech

Researchers from RMIT University in Melbourne, Australia have demonstrated for the first time a working rechargeable "proton battery" that could re-wire how we power our homes, vehicles and devices.
Mar 7, 2018
11
5656
Energy & Green Tech

Electrolytic hydrogen production entails the generation of hydrogen from water using electrical power, which should ideally come from renewable power sources such as sunlight and wind. Although this method of producing hydrogen ...
Energy & Green Tech

Hydrogen has long been touted as the future for passenger cars. The hydrogen fuel cell electric vehicle (FCEV), which simply runs on pressurised hydrogen from a fuelling station, produces zero carbon emissions from its exhaust. ...
Jun 3, 2020
4
2009
Energy & Green Tech
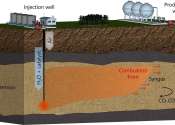
Skoltech researchers have found a way to produce hydrogen from natural gas with 45% efficiency right in the gas field by injecting steam and a catalyst into a well and adding oxygen to ignite the gas. Catalyst-assisted combustion ...
Jun 7, 2024
1
93
Energy & Green Tech

Japan's new 2050 deadline for carbon neutrality has thrown a spotlight on its efforts to find new, greener fuel options, including an ambitious but controversial liquid hydrogen venture.
Nov 2, 2020
1
318
Hydrogen (pronounced /ˈhaɪdrədʒən/) is the chemical element with atomic number 1. It is represented by the symbol H. At standard temperature and pressure, hydrogen is a colorless, odorless, nonmetallic, tasteless, highly flammable diatomic gas with the molecular formula H2. With an atomic weight of 1.00794 u, hydrogen is the lightest element.
Hydrogen is the most abundant chemical element, constituting roughly 75% of the universe's elemental mass. Stars in the main sequence are mainly composed of hydrogen in its plasma state. Elemental hydrogen is relatively rare on Earth. Industrial production is from hydrocarbons such as methane with most being used "captively" at the production site. The two largest uses are in fossil fuel processing (e.g., hydrocracking) and ammonia production mostly for the fertilizer market. Hydrogen may be produced from water by electrolysis at substantially greater cost than production from natural gas.
The most common isotope of hydrogen is protium (name rarely used, symbol H) with a single proton and no neutrons. In ionic compounds it can take a negative charge (an anion known as a hydride and written as H−), or as a positively-charged species H+. The latter cation is written as though composed of a bare proton, but in reality, hydrogen cations in ionic compounds always occur as more complex species. Hydrogen forms compounds with most elements and is present in water and most organic compounds. It plays a particularly important role in acid-base chemistry with many reactions exchanging protons between soluble molecules. As the only neutral atom with an analytic solution to the Schrödinger equation, the study of the energetics and bonding of the hydrogen atom played a key role in the development of quantum mechanics.
Hydrogen is important in metallurgy as it can embrittle many metals, complicating the design of pipelines and storage tanks. Hydrogen is highly soluble in many rare earth and transition metals and is soluble in both nanocrystalline and amorphous metals. Hydrogen solubility in metals is influenced by local distortions or impurities in the crystal lattice.
This text uses material from Wikipedia, licensed under CC BY-SA