This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new system for producing green hydrogen cheaply and efficiently
What does it take to produce green hydrogen more efficiently and cheaply? Apparently, small ruthenium particles and a solar-powered system for water electrolysis. This is the solution proposed by a joint team involving the Istituto Italiano di Tecnologia (Italian Institute of Technology, IIT) of Genoa, and BeDimensional S.p.A. (an IIT spin-off).
The technology, developed in the context of the Joint-lab's activities and recently published in Nature Communications and the Journal of the American Chemical Society, is based on a new family of electrocatalysts that could reduce the costs of green hydrogen production on an industrial scale.
Hydrogen is considered a sustainable energy vector, an alternative to fossil fuels. But not all hydrogen is the same when it comes to environmental impact. Indeed, the main way hydrogen is produced nowadays is through methane steam reforming, a fossil fuel-based process that releases carbon dioxide (CO2) as a by-product. The hydrogen produced by this process is classified as "gray" (when CO2 is released into the atmosphere) or "blue" (when CO2 undergoes capture and geological storage).
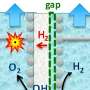
To significantly reduce emissions to zero by 2050, these processes must be replaced with more environmentally sustainable ones that deliver "green" (i.e., net-zero emissions) hydrogen. The cost of "green" hydrogen critically depends on the energy efficiency of the setup (the electrolyzer) that splits water molecules into hydrogen and oxygen.
The researchers from the joint team of this discovery have developed a new method that guarantees greater efficiency than currently known methods in the conversion of electrical energy (the energy bias exploited to split water molecules) into the chemical energy stored in the hydrogen molecules that are produced. The team has developed a concept of a catalyst and has used renewable energy sources, such as the electrical energy produced by a solar panel.
"In our study, we have shown how it is possible to maximize the efficiency of a robust, well-developed technology despite an initial investment that is slightly greater than what would be needed for a standard electrolyzer. This is because we are using a precious metal such as ruthenium," said Yong Zuo and Michele Ferri from the Nanochemistry Group at IIT in Genoa.
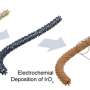
The researchers used nanoparticles of ruthenium, a noble metal that is similar to platinum in its chemical behavior but far cheaper. Ruthenium nanoparticles serve as the active phase of the electrolyzer's cathode, leading to an increased efficiency of the overall electrolyzer
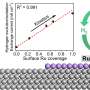
"We have run electrochemical analyses and tests under industrially significant conditions that have enabled us to assess the catalytic activity of our materials. Additionally, theoretical simulations allowed us to understand the catalytic behavior of ruthenium nanoparticles at the molecular level; in other words, the mechanism of water splitting on their surfaces," explained Sebastiano Bellani and Marilena Zappia from BeDimensional, who were involved in the discovery.
"Combining the data from our experiments with additional process parameters, we have carried out a techno-economic analysis that demonstrated the competitiveness of this technology when compared to state-of-the-art electrolyzers."
Ruthenium is a precious metal that is obtained in small quantities as a by-product of platinum extraction (30 tons per year, as compared to the annual production of 200 tons of platinum) but at a lower cost (18.5 dollars per gram as opposed to 30 dollars for platinum). The new technology involves the use of just 40 mg of ruthenium per kilowatt, in stark contrast with the extensive use of platinum (up to 1 gram per kilowatt) and iridium (between 1 and 2.5 grams per kilowatt, with iridium price being around 150 dollars per gram) that characterize proton-exchange membrane electrolyzers.
By using ruthenium, the researchers at IIT and BeDimensional have improved the efficiency of alkaline electrolyzers, a technology that has been used for decades due to its robustness and durability.
For example, this technology was on board of the Apollo 11 capsule that brought humanity to the moon in 1969. The new family of ruthenium-based cathodes for alkaline electrolyzers that has been developed is very efficient and has a long operating life, being therefore capable of reducing the production costs of green hydrogen.
"In the future, we plan to apply this and other technologies, such as nanostructured catalysts based on sustainable two-dimensional materials, in up-scaled electrolyzers powered by electrical energy from renewable sources, including electricity produced by photovoltaic panels," concluded the researchers.
More information: Yong Zuo et al, Ru–Cu Nanoheterostructures for Efficient Hydrogen Evolution Reaction in Alkaline Water Electrolyzers, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c06726
Yong Zuo et al, High-performance alkaline water electrolyzers based on Ru-perturbed Cu nanoplatelets cathode, Nature Communications (2023). DOI: 10.1038/s41467-023-40319-5