July 26, 2016 feature
High-voltage lithium-ion battery realized with superconcentrated electrolyte
(Tech Xplore)—One of the biggest challenges facing next-generation lithium-ion batteries in electric vehicles is finding ways to further increase the driving range. One way to do this is by increasing the battery voltage from the present 4 volts to 5 volts. The problem, however, is that the higher voltage is usually accompanied by a severe capacity loss of more than 50% after only 100 charge/discharge cycles.
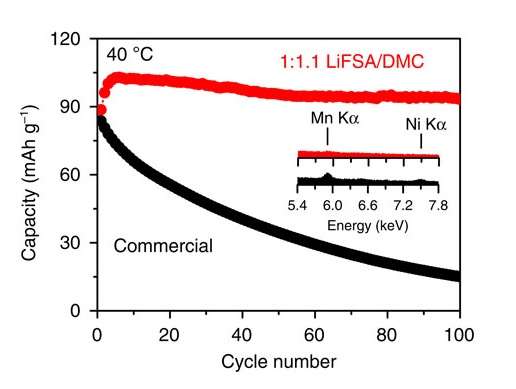
Now in a new study, researchers have found a way to overcome the capacity loss by developing a 5-volt lithium-ion battery that can maintain more than 90% of its capacity over 100 cycles using a superconcentrated lithium-based electrolyte.
The researchers, Jianhui Wang et al., at the University of Tokyo, Kyoto University, JST PRESTO, and the National Institute for Materials Science, all in Japan, have published a paper on the superconcentrated electrolyte in a recent issue of Nature Communications.
"The 5-volt battery using the superconcentrated electrolyte has much higher energy density than current 4-volt batteries while achieving a comparable power density," coauthor Atsuo Yamada, a professor at the University of Tokyo, told Tech Xplore.
He explained that the 5-volt battery's smaller size and weight also contribute to a longer driving range in electric vehicles.
"A battery pack for electric vehicles is composed of many battery cells connected in series to obtain a high voltage (for example, several hundred volts) required to drive the car," he said. "The use of higher-voltage battery cells can reduce the number of serially connected cells, thus decreasing the size and weight of the whole battery pack, which can extend the driving range of the electric vehicles."
In general, the capacity loss that plagues most high-voltage lithium-ion batteries developed to date results from a tradeoff related to the electrolyte salt stability. A highly stable electrolyte salt has the advantage of suppressing the dissolution of the battery's transition metal electrode, but also has the disadvantage of accelerating the dissolution of the battery's aluminum current collector. An unstable electrolyte salt (for example, the widely used LiPF6) has the exact opposite effects. In either case, the dissolution results in severe capacity loss.
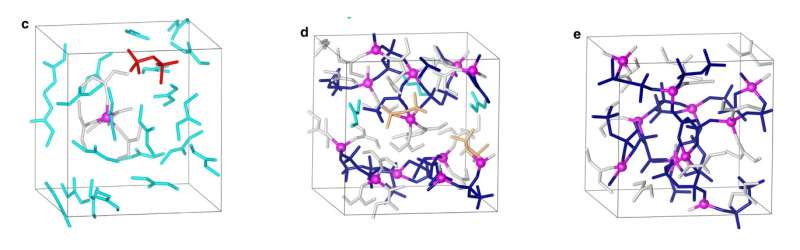
In the new study, the researchers have overcome this electrolyte tradeoff by mixing a stable lithium salt (LiN[SO2F]2 and related formulas) in a solvent at an extremely high concentration of 1:1.1, so that nearly half of the solution is lithium salt. Whereas a slightly less concentrated solution of 1:2 still suffers from unwanted aluminum dissolution and high capacity loss, the superconcentrated solution manages to overcome the electrolyte tradeoff due to its unusual 3D liquid structure.
The highly concentration solution has what the researchers describe as a reinforced 3D network, in contrast to less concentrated solutions where the extra solvent divides the solution structure into smaller parts. The advantage of the reinforced structure is that the negatively charged atoms and ions in the solution—which typically bond with and dissolve the positively charged aluminum ions in the current collector—instead are all bound to the positively charged lithium ions in the electrolyte. Even if a few of the negatively charged atoms and ions manage to avoid being bonded to lithium ions, it's difficult for them to be transported through the dense structure to bind with aluminum ions. Overall, these characteristics greatly suppress the dissolution of the aluminum current collector and lead to the high capacity retention.
Another advantage of the new electrolyte is its enhanced safety due to its much higher thermal stability compared to more dilute electrolytes. The better stability is due to its lower content of organic solvents, since organic solvents create a safety hazard because of their high volatility and flammability.
One drawback of the superconcentrated electrolyte is that it's somewhat more expensive, although the researchers don't see this as a major problem.
"At present, the biggest challenge is materials cost, because the LiN(SO2F)2 salt is currently more expensive than currently used LiPF6 salt," Yamada said. "However, the mass production of the LiN(SO2F)2 salt has recently been initiated and is becoming increasingly available at much lower cost. Hence, we expect that the cost will not be a problem in the future. Moreover, the cost of electrolyte is only less than 7% of the total battery price."
In the future, the researchers plan to further improve the battery performance and try to better understand the chemistry of the superconcentrated electrolyte.
"We are trying to develop new electrolyte systems to further increase the energy density and power density of lithium-ion batteries," Yamada said. "Furthermore, we are interested in various unusual features of the superconcentrated electrolytes which are not shared by conventional dilute electrolytes. We will study the origin of the unusual features and consider their fascinating applications."
More information: Jianhui Wang et al. "Superconcentrated electrolytes for a high-voltage lithium-ion battery." Nature Communications. DOI: 10.1038/ncomms12032
© 2016 Tech Xplore