Significant progress in lithium-air battery development
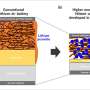
![i) Galvanostatic cycling of Li|electrolyte|Li coin cells containing 0.7 mol dm−3 Li[TFSI] in DMSO (red trace) and in (0.6)DMSO–(0.4)[Pyrr14][TFSI] (blue trace). Inset zooms in on stable appearance of plating stripping in IL/solvent/Li‐salt ternary mixture. ii) Ternary plot showing the formulations of DMSO, Li[TFSI], and [Pyrr14][TFSI] studied in this work. iii–vi) Normalized voltage (E/ET1) profiles of Li|electrolyte|Li symmetrical cells for the A(1:0), B(9:1), C(6:4), and D(6:4) binary/ternary solutions, respectively. Bracketed numbers highlight the molar ratio of DMSO to Li+ in each solution. Cells were cycled at j = ±0.05 mA cm−2 for 1 h per half‐cycle (Q = 0.05 mAh cm−2 per half‐cycle). Significant progress in lithium-air battery development](https://scx1.b-cdn.net/csz/news/800a/2021/significant-progress-i.jpg)
Research led by the University of Liverpool, in partnership with Johnson Matthey PLC and Loughborough University, is making significant progress in the development of stable and practical electrolytes for lithium-oxygen batteries.
The lithium-oxygen (Li-O2) battery (or lithium-air battery), consisting of Li-metal and a porous conductive framework as its electrode's releases energy from the reaction of oxygen from the air and lithium. The technology is in its infancy, but in theory could provide much greater energy storage than the conventional lithium-ion battery.
In a paper published in the journal Advanced Functional Materials, Professor Laurence Hardwick from the University of Liverpool's Stephenson Institute for Renewable Energy (SIRE) and colleagues meticulously characterized and developed electrolyte formulations that significantly minimizes side reactions within the battery to enable improved longer cycle stability.
According to lead author of the paper, Dr. Alex Neale who is also with SIRE, the research demonstrates that the reactivity of certain electrolyte components can be switched off by precise control of component ratios.
Dr. Neale said: "The ability to precisely formulate the electrolyte using readily-available, low volatility components enabled us to specially tailor an electrolyte for the needs of metal-air battery technology that delivered greatly improved cycle stability and functionality."
"The outcomes from our study really show that by understanding the precise coordination environment of the lithium ion within our electrolytes, we can link this directly to achieving significant gains in electrolyte stability at the Li metal electrode interface and, consequently, enhancements in actual cell performance."
Dr. Pooja Goddard from Loughborough University's Department of Chemistry said: "It was exciting to see through the use of both calculations and experimental data we were able to identify the key physical parameters that enabled the formulations to become stable against the lithium metal electrode interface."
The designed electrolytes provide new benchmark formulations that will support ongoing investigations within our research groups to understand and develop new, and practically viable, cathode architectures to reduce round-trip inefficiencies and further extend cycle lifetimes.
Enrico Petrucco from Johnson Matthey PLC said "This work exemplifies a useful electrolyte design strategy for Li-air batteries underpinned with excellent science within a great collaboration. This moves us another step closer towards practical routes to overcome complex Li-air challenges."
The collaborative research between the two University research groups in Liverpool and Loughborough and Johnson Matthey PLC was made possible by support from an Innovate UK Grant that enables industry and academia to work together to tackle technology focused research challenges.
More information: Alex R. Neale et al. Design Parameters for Ionic Liquid–Molecular Solvent Blend Electrolytes to Enable Stable Li Metal Cycling Within Li–O 2 Batteries, Advanced Functional Materials (2021). DOI: 10.1002/adfm.202010627