A new look at the problem of energy efficiency in lithium-ion batteries
An international research team featuring two Skoltech scientists has experimentally demonstrated that a long-standing explanation for low energy efficiency in lithium-ion batteries does not hold. The researchers explained the phenomenon in terms of slow electron transfer between oxygen and transition metal atoms in the cathode, rather than the atoms themselves undergoing migration. The study came out Thursday in the journal Nature Chemistry.
The lithium-ion batteries used in electric vehicles and gadgets today have about half the capacity their cousins with lithium-enriched oxide cathodes could deliver. The problem with the latter technology is it has low efficiency: You have to spend significantly more power to charge up the battery than it will ultimately provide. Over time, and particularly for applications consuming much energy, this lost power really adds up, making that type of batteries commercially not viable as of now.
To unlock the potential of the batteries with lithium-enriched oxide cathodes, researchers have to understand the mechanism behind their inefficiency and exactly where the lost energy goes. The recent study in Nature Chemistry provides experimental evidence refuting the previously held explanation of the phenomenon—technically known as voltage hysteresis—and offers a new theory to account for it.
As a lithium-ion battery gets charged, lithium ions travel between its two electrodes. Migrating toward the anode, they leave behind vacancies in the cathode. The other half of the cycle involves lithium ions going back as the energy gets expended, say to power a phone.
"In the meantime, however, some of the transition metal atoms making up the cathode might have temporarily invaded the vacancies and then pulled back again, spending valuable energy on this jumping around. Or so the old theory of voltage hysteresis went," study co-author and Skoltech Ph.D. student Anatoly Morozov said.
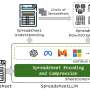
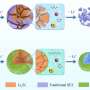
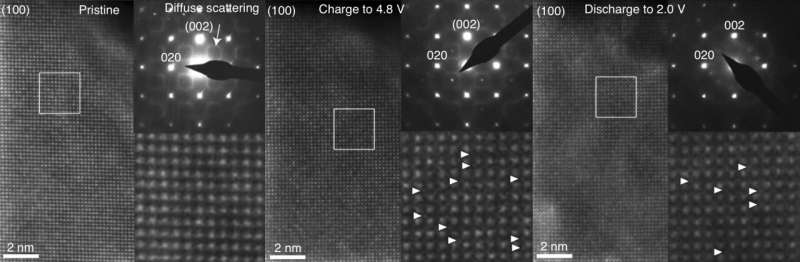
To test this explanation, the researchers used a transmission electron microscope at Skoltech's Advanced Imaging Core Facility to monitor the atomic structure of a lithium-enriched battery cathode made of a material with the formula Li1.17Ti0.33Fe0.5O2 at different stages in the battery's charge-discharge cycle (see the image below). However, no significant migration of iron or titanium atoms to lithium vacancies was observed, suggesting that some other process was siphoning power.
"Our findings inspired the team to seek the origin of voltage hysteresis elsewhere. What gives rise to the phenomenon is not reversible cation migration but rather the reversible transfer of electrons between the atoms of oxygen and transition metals. As the battery gets charged, some of the electrons from iron are hijacked by the oxygen atoms. Later on, they go back. This reversible transfer consumes some of the energy," explained Professor Artem Abakumov, who heads the Center of Energy Science and Technology at Skoltech.
"Understanding voltage hysteresis in terms of electron transfer might have immediate implications for mitigating this unwelcome effect to enable next-generation lithium-ion batteries with record-high energy density for powering electric cars and portable electronics," he went on. "To enable that next step, chemists could manipulate the electron transfer barriers by varying the covalency of the cation-anion bonding, guided by the periodic table and such concepts as 'chemical softness.'"
"This demonstrates the power of advanced transmission electron microscopy for deciphering local structures of extreme complexity. It is really great that young researchers at Skoltech have direct and easy access to such sophisticated equipment as aberration-corrected electron microscopes, and opportunities for further training. This enables us to contribute to top-level battery research in collaboration with our international peers in both academia and the industry," Morozov added.
More information: Biao Li et al, Correlating ligand-to-metal charge transfer with voltage hysteresis in a Li-rich rock-salt compound exhibiting anionic redox, Nature Chemistry (2021). DOI: 10.1038/s41557-021-00775-2