December 1, 2022 report
Electrolyzing seawater without side-reactions, corrosion problems or the need for purifying the water first

A team of researchers affiliated with multiple institutions in China has developed a type of electrolysis that works with native seawater and does not have side-reactions or corrosion problems. In their study, published in the journal Nature, the group tested their process in a real-world location. Heping Xie, with Shenzhen University, has published a Research Briefing in the same journal issue outlining this new effort.
As scientists have become aware of the problems associated with burning fossil fuels, they have turned their attention to alternative sources of fuel for generating power. One possibility is hydrogen, a gas that can be burned without generating harmful greenhouse gases. However, hydrogen has posed problems involved in generating and storing it.
Electrolysis is one method of generating hydrogen, applying electricity to water to split it into its oxygen and hydrogen components. Unfortunately, current electrolysis methods require nearly pure water. In this new effort, the researchers developed an electrolysis process using seawater, a source that is a long way from pure.
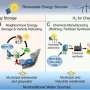
The process involves a membrane similar to the type used in waterproof clothing. It has pores that are tiny enough to allow individual molecules through, but prohibits molecules bunched together, as is the case with water. In their device, the outer part of the membrane is exposed to seawater while the inner side is exposed to a small amount of potassium hydroxide (KOH). Inside of the pouch that holds the KOH, the team placed electrodes that generate hydrogen and oxygen on both sides of a separator, which keeps the gas streams clean.
In action, as the device was dipped into seawater, water inside was split, producing hydrogen and oxygen. That reduced the concentration levels of KOH, which pulled more seawater into the device, allowing it to run continuously. In the device, a small amount of seawater was held in a vapor state, which allowed it to pass cleanly through the membrane, where it once again reverted to its water state, providing pure water for electrolysis. Tubes vented the oxygen to collect the hydrogen.
The researchers tested their device in Shenzhen Bay (just north of Hong Kong). Measurements showed it capable of making as much hydrogen as conventional methods, and it was also hardy—it was run for 3,200 hours without signs of degradation.
More information: Heping Xie et al, A membrane-based seawater electrolyser for hydrogen generation, Nature (2022). DOI: 10.1038/s41586-022-05379-5
A practical method for splitting seawater into hydrogen fuel, Nature (2022). DOI: 10.1038/d41586-022-03601-y
© 2022 Science X Network