This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Progress toward greener redox-organic electrode batteries
Our modern rechargeable batteries, such as lithium-ion batteries, are anything but sustainable. One alternative is organic batteries with redox-organic electrode materials (OEMs), which can be synthesized from natural "green" materials. In the journal Angewandte Chemie, a Chinese team has now introduced a new OEM for aqueous organic high-capacity batteries that can be easily and cheaply recycled.
Traditional inorganic electrode materials in commercial batteries involve a whole spectrum of problems: limited resources, toxic elements, environmental problems, partly unacceptable mining conditions, limited capacity, difficulties in recycling, and high costs. No sustainable batteries can be developed on a large scale based on these electrodes, though they are needed for an energy transition.
Organic batteries with OEMs are still at the very beginning of their long road toward practical application. A team led by Chengliang Wang at Huazhong University of Science and Technology has now taken a significant step in this direction. The goal is to use OEMs in batteries with aqueous electrolytes. These are "greener", more sustainable, and less expensive than the conventional organic electrolytes in lithium-ion batteries.
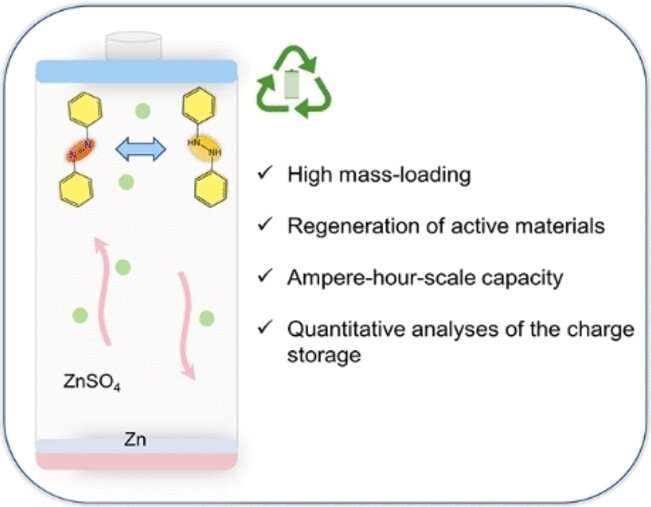
The team chose to use azobenzene, a material that can be produced inexpensively on a large scale and is insoluble in water while being highly soluble in organic solvents. Whereas most other functional groups can only transfer one electron, the azo group (–N=N–) in this molecule is able to reversibly transfer two electrons, which contributes to a high capacity.
Comprehensive analyses demonstrated that, during the discharge process, the azobenzene is converted to hydroazobenzene after absorbing two of the electrons—through the rapid, reversible binding of two protons (H+). Prototype coin cells and laminated pouch cells of various sizes with azobenzene OEMs and zinc counter-electrodes reached capacities on the scale of ampere hours, which were retained over 200 charge/discharge cycles.
In contrast to polymeric OEMs, the small azobenzene molecules can be inexpensively recycled with a simple extraction using commercial organic solvents. The electrode material is air stable in both its charged and discharged states and can be recycled in yields of over 90% in every state of charge. The recycled products could be directly reused as OEMs with no loss of capacity.
More information: Yuan Chen et al, A Recyclable and Scalable High‐Capacity Organic Battery, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202302539