This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cactus plant inspires cost-effective hydrogen production
More than 100 years ago, scientists discovered how to turn water into hydrogen gas—a highly desired green energy that's been nicknamed "the fuel of the future."
Despite this breakthrough, hydrogen has not latched on as a dominant fuel source. Breaking down water into hydrogen can be inefficient and costly and the transformation process, called electrolysis, remains unperfected.
Now, engineers at The University of Texas at El Paso have proposed a low-cost, nickel-based material to help split water more cheaply and efficiently. Their inspiration? A desert succulent known as the prickly pear cactus. The material is described in a new paper in the journal ACS Applied Materials & Interfaces.
"This is nature-inspired design in the laboratory," said UTEP Mechanical Engineering Professor Ramana Chintalapalle, Ph.D., who led the study. "You have this plant with an extensive surface that can absorb moisture and survive in extreme environments. We thought, 'How can we incorporate this into our research?'"
The hydrogen problem
Electrolysis is the process of splitting water with electricity and an electrocatalyst—a material that speeds up any chemical reaction. Current techniques to split water rely heavily on platinum as a catalyst, which has its drawbacks.
"Platinum is the dominant material used to help split water, but it is very expensive—more expensive than gold—and it's just not feasible to use it on a large scale because of its price," Chintalapalle explained. "We need a catalyst that's more economically viable so every country can reasonably adopt hydrogen."
A prickly solution
Navid Attarzadeh first noticed the prickly pear cactus while walking to UTEP's Center for Advanced Materials Research lab. The team had been exploring nickel as a catalytic replacement for platinum, a metal that is abundant on Earth and 1,000 times cheaper than platinum.
Nickel, however, is not as quick and effective at breaking down water into hydrogen.
"Every day, I passed this same plant," said Attarzadeh, a doctoral student in environmental science and engineering. "And I started connecting it to our catalyst problem. What caught my attention was how big the leaves and fruits were compared to other desert plants; the prickly pear has an extraordinary surface area."
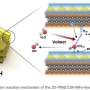
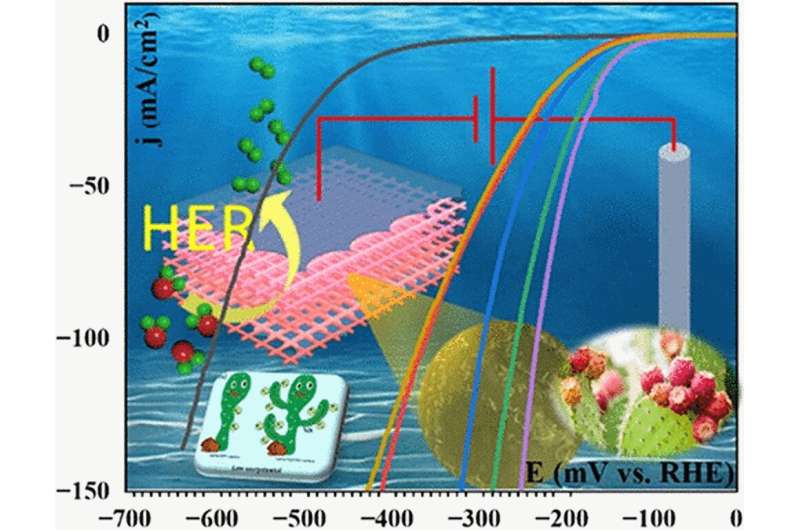
That's when the Attarzadeh had an idea. What if they designed a 3D nickel-based catalyst in the shape of the prickly pear cactus? The larger surface area could accommodate more electrochemical reactions—creating more hydrogen than nickel typically can.
The team quickly designed the nano-scale structure—invisible to the human eye—and put it to the test.
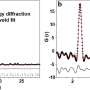
"We tested the catalyst's ability to split water repeatedly and had good results," Chintalapalle said.
He added that this is a fundamental discovery and the process needs further refinement, but it's a step in the right direction.
"Hydrogen gas can transform energy technology for our country—without generating greenhouse gas emissions," Chintalapalle said. "Our carbon footprint could be eliminated; we need to keep pursuing this."
More information: Navid Attarzadeh et al, Nature-Inspired Design of Nano-Architecture-Aligned Ni5P4-Ni2P/NiS Arrays for Enhanced Electrocatalytic Activity of Hydrogen Evolution Reaction (HER), ACS Applied Materials & Interfaces (2023). DOI: 10.1021/acsami.3c00781