This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Using our oceans to fight climate change
The ocean isn't just impacted by climate change—it may also be part of the solution to reversing it.
Direct ocean carbon capture (DOC) is an emerging form of negative emissions technology that has advantages over its on-land counterpart, direct air capture, because of its ability to avoid land use. DOC can also conveniently be paired with offshore wind and offshore carbon dioxide storage.
Katherine Hornbostel, an assistant professor of mechanical engineering and materials science at the University of Pittsburgh Swanson School of Engineering, is well-versed in the field of carbon capture technologies. She has been actively collaborating with Assistant Professor Tagbo Niepa from Pitt's Department of Chemical and Petroleum Engineering to develop innovative ocean carbon capture solutions.
The team published two sister papers, "Demonstration of direct ocean carbon capture using encapsulated solvents" and "Demonstration of direct ocean carbon capture using hollow fiber membrane contactors," in the Chemical Engineering Journal. These two papers demonstrate experimentally and computationally how two types of membrane contactors—encapsulated solvents and hollow fiber membrane contactors—can remove carbon dioxide from the ocean.
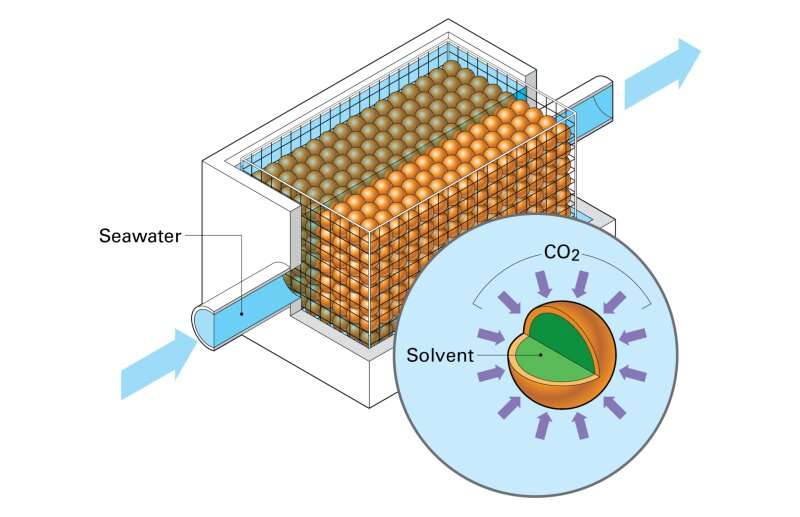
"Membrane contactors are just what they sound like," Hornbostel said. "They're membranes that bring two fluids into contact with each other. In this case, we're bringing together ocean water on one side and a solvent on the other."
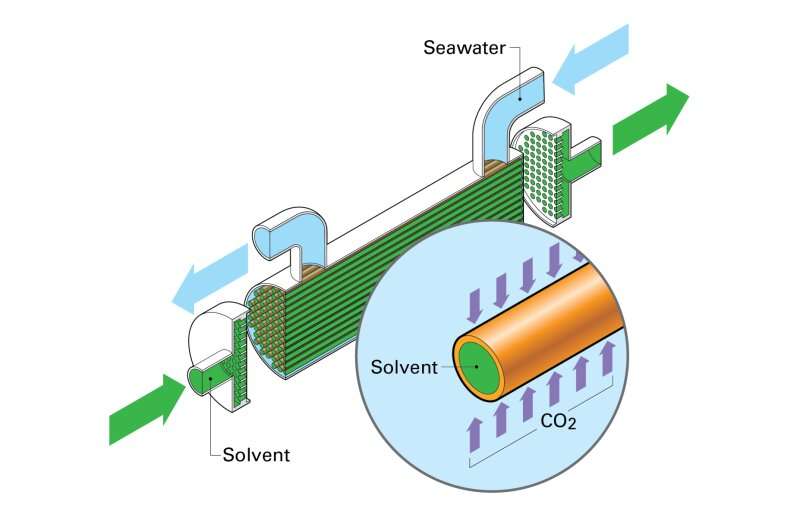
The team tested two types of membrane contactors: hollow fiber and encapsulated solvents. The biggest difference between the two technologies is their shape. While hollow fiber membrane contactors look like straws, encapsulated solvents look like caviar. Otherwise, they work exactly the same.
"The idea with both is to get a really high surface area of contact between seawater and solvent," Hornbostel explained. "The more surface area you have, the better the carbon dioxide removal rate."
Swinging the seawater
Carbon dioxide will want to travel across the membrane towards the solvent, made from a sodium solution that reacts with carbon dioxide. When seawater comes into contact with the solvent, the carbon dioxide will react and separate from the seawater. The solution then has to be re-circulated to make the process more cost effective—something the team is still working to improve.
"Theoretically, we could significantly lower the price if we could swing the pH of the seawater side," Hornbostel said. "Carbon dioxide is not typically available in seawater at its baseline level of pH, so you have to swing the pH lower in the seawater to make it more acidic and then more carbon dioxide bubbles off."
Hornbostel's team is currently pursuing methods for swinging seawater pH with membrane surface treatments and investigating coupling direct ocean capture with desalination to lower system costs.
More information: Austin Lieber et al, Demonstration of direct ocean carbon capture using encapsulated solvents, Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.144140
Joanna Rivero et al, Demonstration of direct ocean carbon capture using hollow fiber membrane contactors, Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.143868