This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chloride ions from seawater could replace lithium in batteries of the future
Sodium, potassium and zinc have all been promising contenders for lithium's place in rechargeable batteries of the future, but researchers at Worcester Polytechnic Institute (WPI) have added an unusual and more abundant competitor to the mix: chloride, the richest negatively charged ions in seawater.
Xiaowei Teng, the James H. Manning professor of Chemical Engineering at WPI, has discovered a new redox chemistry empowered by chloride ions for the development of seawater green batteries.
Modern lithium-ion batteries used in various applications, including electric vehicles, can be problematic for grid storage, given their high cost and reliance on critical materials, such as cobalt, nickel, and lithium, as well as their limited geographical availability. For example, six countries own over 85% of lithium reserves on the land.
Teng and his research collaborators—Heath Turner, professor of Chemical and Biological Engineering at the University of Alabama, and Lihua Zhang, Milinda Abeykoon, Gihan Kwon, Daniel Olds, all research scientists at Brookhaven National Laboratory in New York—went beyond the limits of current green battery technology by leveraging chloride ions to empower redox chemistry of iron oxide battery materials.
Teng and his colleagues reported on the new battery chemistry in "Chloride-Insertion Enhances the Electrochemical Oxidation of Iron Hydroxide Double Layer Hydroxide into Oxyhydroxide in Alkaline Iron Batteries", a paper published in Chemistry of Materials.
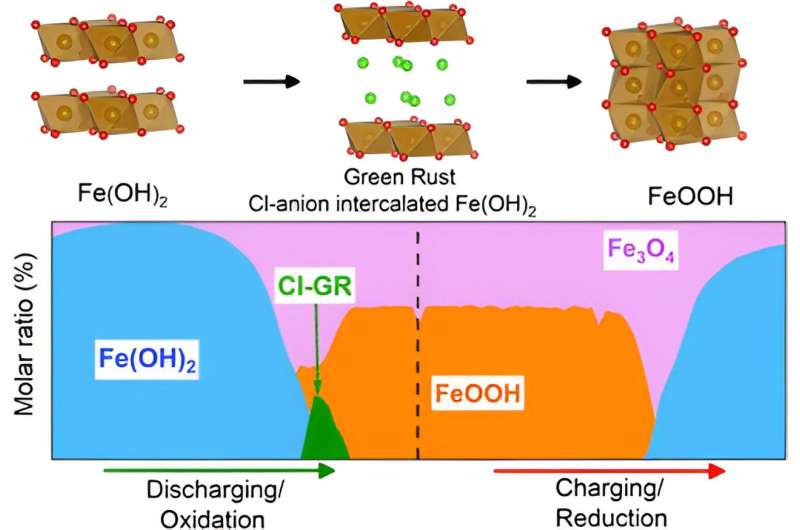
This study revealed that chloride ion insertion into Fe(OH)2 layered double hydroxide formed a Green Rust intermediate crystalline material, which assisted a one-charge transfer Fe(OH)2/FeOOH conversion reaction and improved cycling stability. This new iron redox chemistry was discovered and examined in the WPI lab. Teng and his graduate student Sathya Narayanan Jagadeesan, who is the leading author of the article, further traveled to Department of Energy User Facilities at Brookhaven National Laboratory to conduct experiments to validate the results using operando synchrotron X-ray diffraction and high-resolution elementary mapping.
Teng and his WPI team made an aqueous battery, a small lab-scale prototype that operated in the water-based electrolyte, using electrodes made mostly from abundant elements such as iron oxides and hydroxides. While the team hasn't calculated the cost, the use of earth-abundant materials should tip the scale in their favor, Teng says. The U.S. produces over 15 million tons of scrap iron wastes that are not recycled each year, many of which exist in the form of rust. Therefore, the reported rechargeable alkaline iron battery chemistry helps repurpose the iron rust waste materials for modern energy storage.
More information: Sathya Narayanan Jagadeesan et al, Chloride Insertion Enhances the Electrochemical Oxidation of Iron Hydroxide Double-Layer Hydroxide into Oxyhydroxide in Alkaline Iron Batteries, Chemistry of Materials (2023). DOI: 10.1021/acs.chemmater.3c01496