This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Metal organic frameworks could turn greenhouse gas into 'gold'
Fluorine can be a beneficial ingredient in medicines because of its excellent pharmacological properties, according to Phillip Milner, assistant professor of chemistry and chemical biology in the College of Arts and Sciences (A&S). But fluorinated chemicals can be difficult—even dangerous—to work with, requiring complex instrumentation.
What's more: fluorinated gases are categorized as greenhouse gases, with the potential to trap heat in Earth's atmosphere.
The Milner Lab has found a way to handle fluorinated gases as stable solids using metal-organic frameworks (MOFs), porous sponge-like materials that stabilize and capture reactive gases. It's an innovative use of MOFs, with a promising side benefit: The same process could someday be used to capture harmful fluorinated emissions and convert them to valuable drug-like molecules or agrochemicals.
"In this way, we can reduce greenhouse gas emissions while making gold out of an otherwise environmentally destructive waste gas," Milner said.
"Handling Fluorinated Gases as Solid Reagents Using Metal-Organic Frameworks" published in Science. The first author is doctoral student Kaitlyn T. Keasler. Co-authors include Mary Zick and Cornell doctoral students Emily E. Stacy and Jaehwan Kim, with Milner as corresponding author.
A specialty of the Milner Lab, metal-organic frameworks can reversibly absorb guest molecules such as gaseous reagents within their pores, Keasler said.
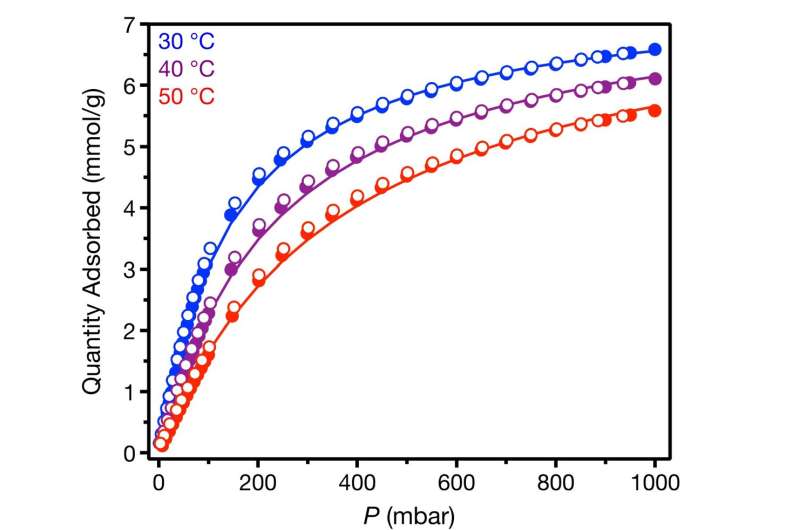
The researchers studied uptake of vinylidene fluoride (VDF), a representative simple fluorinated commodity chemical, by 12 different open-metal-site MOFs. They found an optimal inexpensive MOF that strongly binds vinylidene fluoride with a high capacity, which allows for the minimal amount of MOF to be used to store and deliver the maximum amount of gas.
"We used the MOFs to reversibly store fluorinated gaseous reagents as crystalline solids, which we termed gas-MOF reagents," Keasler said. "The resulting gas–MOF reagents can be handled on the benchtop and stored in air at low temperatures prior to gas release into solution. The use of MOFs enables easy handling of these simple fluorinated gases as solid reagents which can be simply tossed into reactions."
Encapsulating the gas inside of a MOF results in a significant volume reduction, Milner said, The amount of gas that would fill a room can be stored in just a handful of powder.
MOFs have been used to pull water from air and to store hydrogen for zero-emission cars, Milner said, but storing fluorinated gases in these sponge-like materials is new.
"These sponges (MOFs) are not currently used to make pharmaceutical compounds. It's like a separate world," Milner said. "The fact that we might be able to bring them together is a cool direction to go in for our lab."
The newfound ability to bind fluorinated gases strongly in an MOF is an important step toward using the same technology to capture greenhouse gas from industry.
Fluorinated drug molecules are not a major cause of organic pollution, but other industries do release concerning amounts, Milner said. An example is the hydrofluorocarbon fluoroform (CHF3), a byproduct of the manufacture of Teflon. This compound is about 11,000 times more potent than carbon dioxide and a candidate for future capture technology the Milner Lab is working on.
"We've found a way to bind them, but as a pure gas," Milner said. "The next step would be to find a way to pull fluorinated gases from an emissions stream, taking terrible waste gas that's destroying the planet and turn it into something valuable that could save lives. That's the big dream."
More information: Kaitlyn T. Keasler et al, Handling fluorinated gases as solid reagents using metal-organic frameworks, Science (2023). DOI: 10.1126/science.adg8835