October 13, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A promising molecular catalyst for aqueous polysulfide-based redox flow batteries

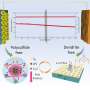
In recent years, researchers have been exploring the potential of a wide range of new battery technologies, including so-called redox flow batteries. Redox flow batteries, also known as flow batteries, are battery cells that generate electricity and store energy via so-called redox chemical reactions.
Polysulfides are active materials that could be particularly promising for the development of flow batteries, due to their low-cost and abundancy on Earth. Despite their advantageous characteristics, however, these materials exhibit a sluggish redox or reduction reaction. This has so far limited both the energy efficiency and power density of polysulfide-based flow batteries.
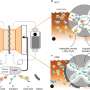
Researchers at The Chinese University of Hong Kong recently introduced a new molecular catalyst that could help to boost the performance of flow batteries based on polysulfides. This catalyst, introduced in Nature Energy, is both durable and active, thus enabling fast redox reactions inside a battery cell.
"The idea of introducing redox-active molecular catalysts first came to us during a brain-storm meeting with the team, in which we were discussing the bottlenecks for the commercialization of polysulfide-based RFBs," Yi-Chun Lu, one of the researchers who carried out the study, told Tech Xplore.
"At that time, we had just finished another project on solving the 'dead MnO2' issue in zinc–manganese batteries using an iodide mediator. Building on these experiences, we speculated that introducing redox-active molecular catalysts could address the kinetics bottleneck of the polysulfide reduction."
Isoalloxazine and quinone are two substances often used in energy applications, as they are known to be efficiently electron shuttles. In living cells, these molecules can proficiently transfer electrons in the so-called respiratory chain, which is what ultimately produces energy. The new molecular catalyst introduced by Lu and his colleagues is based on a compound derived from isoalloxazine, namely riboflavin sodium phosphate (FMN-Na).
"FMN-Na, an isoalloxazine derivative, is an ideal candidate to catalyze polysulfide reduction owing to its fast redox kinetics and suitable redox potential," Lu explained. "During the charging process, the molecular catalyst receives electrons from the electrode and is reduced, forming reduced-FMN. This complex subsequently transfers electrons to polysulfide through a spontaneous chemical reaction during which the molecular catalyst is oxidized back to its original state (oxidized-FMN)."
The design strategy proposed by this team of researchers can improve the electrochemical reduction capabilities of polysulfides, which can in turn facilitate redox reactions inside polysulfide-based battery cells. This is achieved thanks to the rapid redox kinetics of the FMN-Na molecular catalyst they identified.
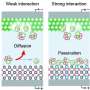
"Most reported catalysts for aqueous polysulfide flow batteries are solid electrocatalysts, which is heterogeneous catalysis using solid substance to catalyst a reaction in the solution (i.e., the polysulfide reduction reaction)," Lu said.
"Different from heterogeneous catalysis, molecular catalysis is not limited to the active sites of the electrode and catalyst surface because both the reactant (S42–) and catalyst (FMN-Na) are solubilized. Our work shows that homogeneous catalysis is an effective approach to addressing the sluggish kinetics of polysulfide."
In experiments, flow battery cells containing the team's catalyst were found to perform well, decaying at a rate of 0.00004% per cycle after running for 2,000 cycles at 40mAcm-2. To demonstrate the catalyst's scalability, Lu and his colleagues used it to create a 100 cm2 cell stack.
As demonstrated by the researchers' initial findings, their approach for improving the performance of polysulfide-based flow batteries could also be scaled up to create larger flow battery systems. Future studies could help to further assess and validate the potential of this approach, by testing the performance of various battery cells containing the FMN-Na catalyst.
"We believe that this approach can be widely applied to other flow systems," Lu added. "We are now working with our industrial partner Luquos Energy Ltd. for further R&D and commercialization."
More information: Jiafeng Lei et al, An active and durable molecular catalyst for aqueous polysulfide-based redox flow batteries, Nature Energy (2023). DOI: 10.1038/s41560-023-01370-0
Jiafeng Lei et al, Towards high-areal-capacity aqueous zinc-manganese batteries: promoting MnO2 dissolution by redox mediators. Energy & Environmental Science(2021). DOI: 10.1039/D1EE01120K.
© 2023 Science X Network