This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Enhancing thermo-electrochemical cell efficiency
The generation of electricity from the human body may not be a superpower at all; rather, it appears to be a commonplace occurrence. Recent research conducted by a POSTECH (Pohang University of Science and Technology) team looks at enhancing the efficiency of a thermo-electrochemical cells capable of generating electricity from the human body's natural temperature.
Their study revolves around the increased efficiency of thermo-electrochemical cells, which can convert wasted thermal energy or body temperature into electricity. The research findings are published in Advanced Functional Materials.
When electricity goes unused in either a household or an industrial setting, it dissipates as thermal energy. The concept of thermal energy harvesting has emerged as a solution to counteract energy depletion and address the climate crisis by harnessing electricity from this waste heat and human body heat. However, these cells, which electrochemically transform heat into electricity, face a significant challenge due to their low energy conversion efficiency and their reliance on precious metal catalysts like platinum, hindering their commercial viability.
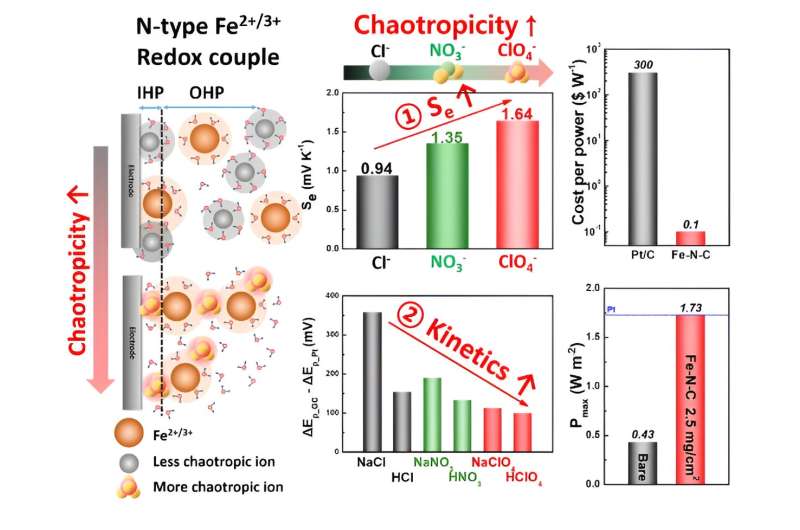
To surmount this limitation, the research team employed an iron-based catalyst along with perchloric acid (CIO4-) anions.
In essence, increasing the chaotropicity within the cell's electrolyte generally results in higher voltage and increased current, thereby enhancing the cell's efficiency. The introduction of perchloric acid was instrumental in augmenting the chaotropicity of the electrolyte, which contained iron ion redox pairs (Fe2+/Fe3+), thus improving the overall efficiency of the cell.
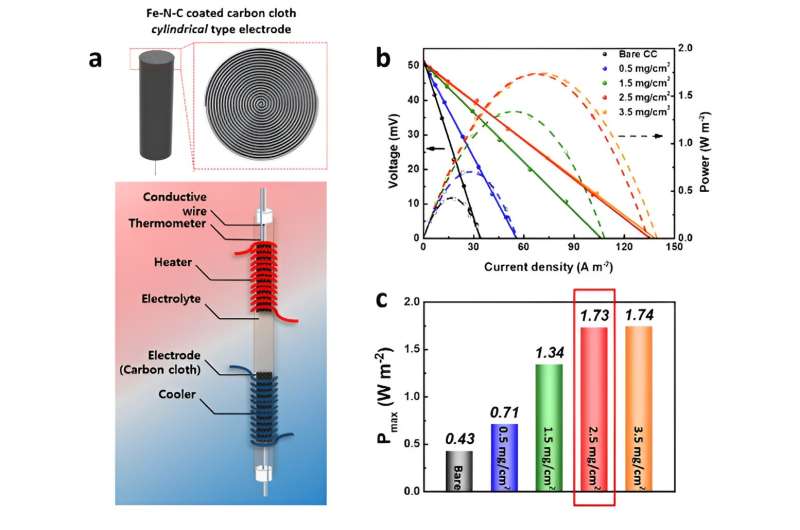
Furthermore, the team introduced a catalyst (Fe-N-C) composed of iron, nitrogen, and carbon to the thermo-electrochemical cell. This catalyst, frequently utilized in hydrogen vehicle fuel cells as an alternative to expensive platinum catalysts, represented an application in a thermo-electrochemical cell for the first time. In test trials, these cells achieved over twice the energy conversion efficiency compared to conventional cells while simultaneously reducing production costs by a factor of 3,000.
The study was led by Professor Yong-Tae Kim from the Department of Materials Science and Engineering and Graduate Institute of Ferrous & Eco Materials Technology, and Dr. Sang-Mun Jung and Seung-Yeon Kang, a master's student, from the Department of Materials Science and Engineering at POSTECH in collaboration with Professor Dongwook Lee from the Department of Materials Science and Engineering at Hongik University.
Professor Yong-Tae Kim commented, "Through our exploration of thermo-electrochemical cell catalysis, a relatively uncharted territory, we have successfully enhanced both efficiency and cost-effectiveness within the system. We anticipate its widespread adoption in energy harvesting applications designed to generate energy from waste heat."
More information: Sang‐Mun Jung et al, Fe─N─C Electrocatalyst for Enhancing Fe(II)/Fe(III) Redox Kinetics in Thermo‐Electrochemical Cells, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202304067