This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researcher leads breakthrough in production of green carbon monoxide using light
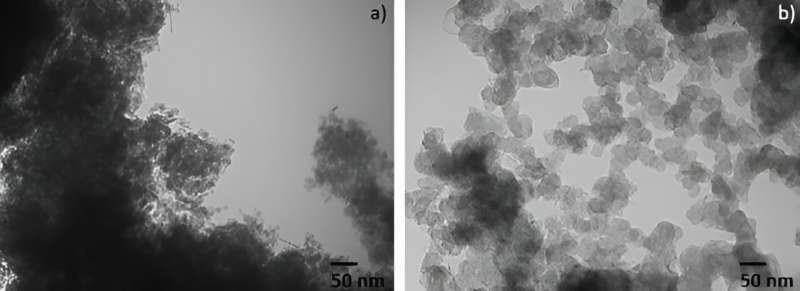
A team of advanced materials chemistry researchers have made a significant breakthrough in the use of light to convert carbon and carbon dioxide (CO2) into carbon monoxide (CO).
In a study published in Energy and Environmental Science, University Professor Geoffrey Ozin of the department of chemistry in the University of Toronto's Faculty of Arts & Science and his team, showcase the process, which represents an alternative to carbon-intensive methods of producing industrial carbon monoxide.
Carbon monoxide and carbon dioxide are both hazardous to human health, with rising carbon dioxide concentrations in our atmosphere increasing the threat of climate change induced by global warming. However, both gases are also vital elements of large-scale production of commodity chemicals and fuels.
In the chemical sector, for example, carbon monoxide serves as feedstock for the synthesis of acetic acid and methanol, pharmaceuticals, fragrance and polymers. In the food industry, it's used for packaging of fresh meat products and to acidify carbonized beverages. Its uses are wide-ranging, from refining or removing rust from metals to serving as a key component of infrared lasers.
Creation of carbon monoxide for such applications is typically done via thermally powered processes involving the gasification of coal and partial oxidation of natural gas, processes that are associated with large carbon footprints and significant toxic byproducts.
Rather than burning fossil fuels to generate carbon monoxide, the greener approach being advanced by Ozin's team utilizes light for the production process, combining this with the emerging practice of using carbon dioxide as chemical feedstock.
The source of carbon to enable this conversion process can be natural, the study shows. It may also come from fossil emission sources as well as air using specialized capture, storage and release technologies, or biochar made by slow burning of agricultural biomass.
The U of T team uses a light-powered reaction that employs this captured carbon dioxide, converting it to carbon monoxide in ways that are less energy- and chemical-intensive than the same reaction driven by heat.
"The CO generated photochemically by this means can justifiably be called green," Ozin said.
A principal investigator in U of T's interdisciplinary Solar Fuels Cluster, Ozin said the research group's vision is to help gradually move the chemical and petrochemical industries away from their reliance on legacy fossil resources to more eco-friendly processes enabled by waste carbon dioxide and carbon, driven by light in solar refineries.
"By this means, it should prove feasible to decarbonize the generation of commodity chemicals and fuels, motivated by the desire to ameliorate greenhouse gas-induced climate change and global warming," Ozin said.
Previous attempts at eco-friendly carbon monoxide production utilized thermal steam-gasification of fossil fuels, biomass or waste materials—super-heating the necessary feedstock with steam to produce the carbon monoxide. However, this generates a large carbon footprint and can be hampered by issues like ash melting and tar contamination. The process requires injections of pure oxygen and produces combustion-related contaminants like dioxins and furans.
Ozin's team is instead spearheading photochemistry methods that can be carried out at room temperature, while generating fewer contaminants.
Green carbon monoxide generation is expected to become more economically viable as advancements are made in the efficiency of photoreactors, battery performance, solar concentration optics and light-emitting diodes—with improvements in these technologies vital to making it competitive with existing thermochemical and electrochemical production methods.
Ultimately, transitioning production of carbon monoxide away from processes that burn fossil fuels toward using renewable energy, unwanted atmospheric carbon monoxide and waste forms of carbon offers the prospect of reducing the environmental footprint of producing this toxic yet vital industrial chemical—while also helping to create green sector jobs.
More information: Camilo J. Viasus Pérez et al, Carbon photochemistry: towards a solar reverse boudouard refinery, Energy & Environmental Science (2023). DOI: 10.1039/D2EE03353D