January 29, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A strategy to realize vertical iontronic energy storage via osmotic effects and electrode redox reactions
In recent years, engineers have been trying to identify new technologies to sustainably generate and store energy. One promising solution leverages the energy produced by osmosis when two fluids with a different salt concentration meet, such as when a freshwater body (e.g., a river) flows into a saltwater body (e.g., the sea).
The energy produced by differences in salt concentration, known as salinity gradient or osmotic energy, has proved very difficult to turn into portable electrical energy in a practical and scalable way. Utilizing this process to power consumer electronics, such as smartwatches and smartbands, thus so far appears impracticable.
Researchers at the Chinese Academy of Sciences, Tsinghua University and Hong Kong University of Science and Technology recently introduced a new method that could help to effectively store iontronic energy based on osmotic effects. This method, outlined in Nature Energy, allowed them to leverage osmotic effects and electrode redox reactions to realize a vertical iontronic energy storage system.
"Almost 10 years ago, we observed an interesting scientific phenomenon, namely that the fast transported ions in water inside graphene oxide (GO) can generate decent energy," Di Wei, co-author of the paper, told Tech Xplore.
"This was the first attempt to provide a very safe energy source that enables new applications including building a foldable energy source on paper and platform for futuristic wearable electronics. Later, further studies attempted to scale up its power via the mathematical fractal design concept as a printing pattern, which unveiled the mechanism using silver (Ag) electrodes."
The recent study by Wei and his collaborators builds on these previous research efforts, drawing inspiration from both the efficient ion-transport dynamics of GO's 2D nanofluidic channels and the carefully tailored interfacial redox reactions. In their paper, the team introduced a new approach to store iontronic energy based on osmotic effects, thus enabling the realization of innovative, renewable, ultrathin, and safe power sources.
"Reverse electrodialysis (RED) is one of the most common methods used for osmotic energy conversion and many investigations have focused on ion-selective membranes to increase the ion transport and lower the internal resistance," Wei explained. "There is a competitive relationship between the selectivity and permeability of the ion-selective membrane, and the ideal optimized thickness is supposed to be less than 1 μm, which is fragile and difficult to obtain."
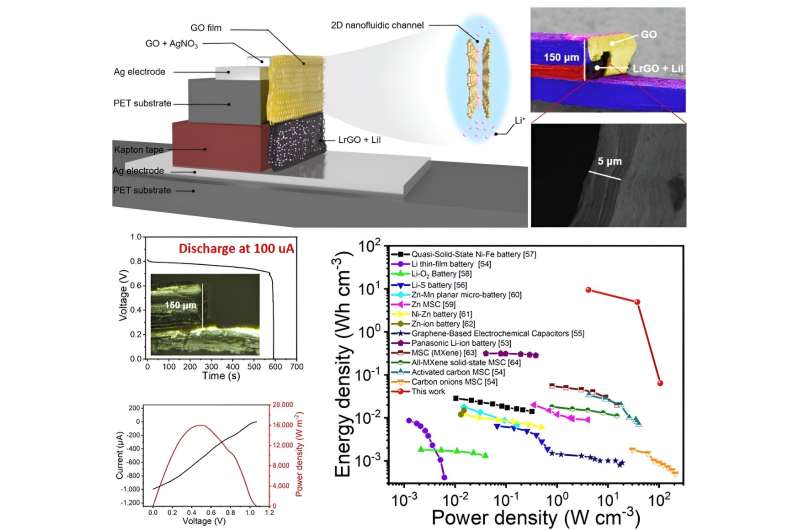
To create their solid-state iontronic energy storage device, Wei and his colleagues first sprayed various GO-based inks onto charge collectors, using an ultrasonic spray-coating system, and then dried these onto a PET substrate. As the inks were drying, the 2D nanofluidic channels of GO started forming.
"The electrode gap of the device, covered by GO, is equal to the thickness of the ion-selective membrane in a conventional osmotic power source," Wei said. "We devised a vertical strategy using the edge of a PET substrate and Kapton film, which offered the capability to scale down the ion-transport distance (equivalent to a decrease in the thickness of the ion-selective membrane)."
In initial tests, the vertical structure realized by the researchers was found to effectively decrease the GO film's internal resistance, thus boosting their device's performance. Notably, the electrode gap employed by Wei and his colleagues in their experiments could potentially also be reduced to the micro-meter or even nano-meter scale, just like the vertical design of those new transistors.
"In contrast to the traditional osmotic energy conversion device, we propose a different approach for preparing a solid-state (i.e., driven by humidity) iontronic energy storage device that utilizes osmotic nanoconfined ion-transport properties and interfacial redox reactions," Wei said. "The vertical structure effectively decreased the internal resistance of the device and showed a superior practical performance due to its enhanced power output with a relatively large GO film area and a shorter ion-transport distance."
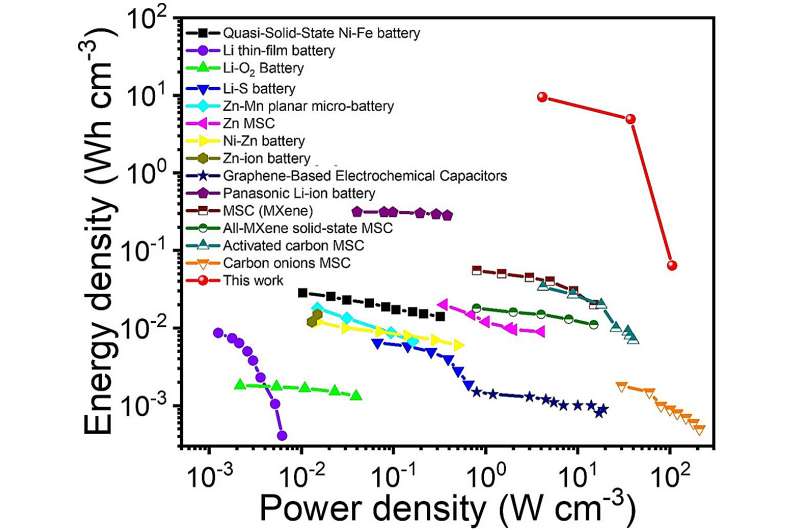
In initial evaluations, the device created by this research team achieved a remarkable, ultrahigh areal output power density of 15,900 W m-2, and the higher volumetric specific energy and power densities, simultaneously. By connecting their devices in series, Wei and his colleagues achieved the corresponding voltage, substantial enough to power commercial electronics.
"Unlike traditional batteries and supercapacitors, our iontronic energy storage device could also be printed directly using commercial coating systems or printers in a cost-effective manner," Wei said.
"Overall, the fundamental understanding of nanoconfined ion dynamics and the rational design of iontronic energy systems may pave the way to investigating the influence of other 2D nanomaterials with various nanohierarchical designs and the fine-tuning of redox reactions in the development of futuristic, highly efficient and renewable power sources."
The recent work by Wei and his collaborators opens interesting avenues for the development of alternative and renewable energy sources for consumer electronics. In the future, the approach outlined in their paper could inspire the development of additional energy storage systems that leverage osmotic effects.
"As indicated in our paper, the fabrication of our device could be scaled up using commercial coating systems or printers in a cost-effective manner," Wei added.
"Because of the volumetric specific energy (9.46 Wh cm−3) and power density (106.33 W cm−3) obtained, the device could power relatively high energy-consumption electronics, such as LCD screens, directly. In our next studies, we will devote ourselves to the applied research of this iontronic energy storage device."
Towards the end of 2023, Wei and his colleagues started conducting preliminary research exploring the potential applications of their iontronic energy storage system. They now plan to continue exploring this research direction, focusing on the potential of their device for powering medical implants, wearable devices and other tiny conformable mobile technologies, such as ultra-thin human-machine interfaces.
More information: Feiyao Yang et al, Vertical iontronic energy storage based on osmotic effects and electrode redox reactions, Nature Energy (2024). DOI: 10.1038/s41560-023-01431-4
Puguang Peng et al, Integratable Paper‐Based Iontronic Power Source for All‐In‐One Disposable Electronics, Advanced Energy Materials (2023). DOI: 10.1002/aenm.202302360
© 2024 Science X Network