This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Unique framework of tin bimetal organic compound facilitates stable lithium-ion storage
Battery capacity is one of the primary bottlenecks in efficient renewable energy storage and significant reductions in carbon emissions. As a battery anode that releases electrons in a lithium-ion battery (LIB), tin (Sn) and Sn-mixture alloys could theoretically store more energy at a higher density than more common carbon-based anodes.
Pairing a Sn-Ti bimetal element with inexpensive ethylene glycol (Sn-Ti-EG) mitigates many of the challenges of using Sn as an anode material and produced an inexpensive LIB with excellent storage and performance characteristics.
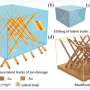
Sn and Sn alloys, or a mixture of another metal with Sn, could outperform other anode materials but suffer from poor stability due to the expansion of the metal during charging and discharging. One way to overcome this limitation is by creating a metal-organic framework (MOF) that maintains rapid electron transfer (energy flow) while providing good stability during charging and recharging.
Materials scientists recently created a Sn-Ti-EG bimetal organic compound MOF that demonstrated high electricity conduction, energy capacity and stability through many charging and discharging cycles.
The researchers published their study in the journal Energy Materials and Devices on November 20.
"Significant efforts have been directed toward developing high-capacity cathode and anode materials for high energy-density LIBs. Because the capacities of well-known cathode materials, for example, LiFePO4, Ni-rich layered oxides and LiMn2O4, have reached their theoretical limits, more attention is being focused on finding anode materials that have high energy densities as a substitute for the commonly used graphite anodes that have a relatively low theoretical capacity and tap density," said Zhen-Dong Huang, senior author of the study and professor in the State Key Laboratory for Organic Electronics and Information Displays & Jiangsu Key Laboratory for Biosensors at Nanjing University of Posts and Telecommunications in Nanjing, China.
Specifically, anodes made from graphite, a crystalline form of carbon, have a theoretical capacity of 372 mAh g-1, which refers to the amount of electric charge (milliampere hours or mAh) the material can deliver per gram (g-1) of that material. In contrast, Sn, bismuth (Bi) and antimony (Sb) metals have higher theoretical capacities than graphite anodes. Sn anodes, for example, have a theoretical capacity of 994 mAh g-1, but suffer from stability issues due to expansion.
"To resolve the stability issues associated with Sn anodes, a myriad of strategies have been explored, including minimizing the particle size, introducing inert metals and assembling with carbon materials. Moreover, rationally designed structures, such as hollow, layered and core–shell structures play an important role in alleviating volume expansion.
"Although these strategies helped the cyclic stability to a certain degree, the… energy densities of the nanostructured Sn-based anodes are normally low. In contrast, metal–organic frameworks have an intrinsically porous structure that not only provides a large number of active sites but also enables rapid electrolyte penetration and electron/ion transfer," said Huang.
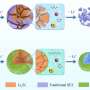
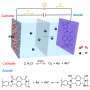
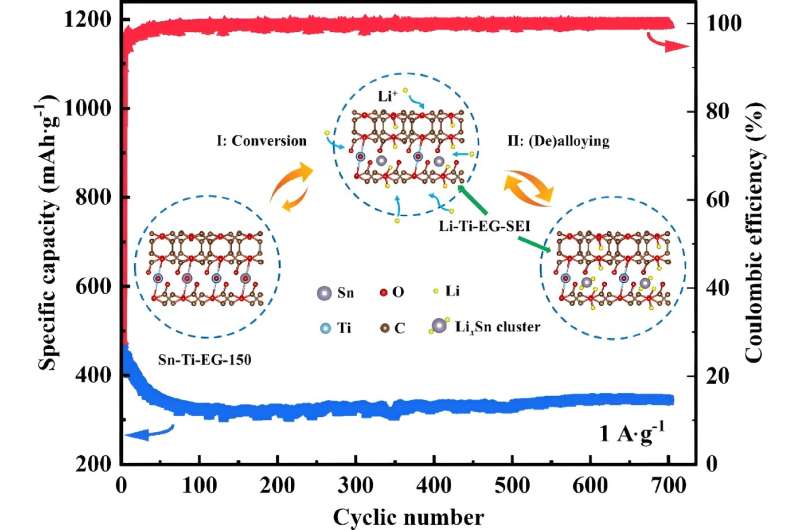
The research team created a unique MOF made up of Sn, Ti and EG that leveraged beneficial characteristics of each component to create a more stable anode material with high electrochemical performance. EG, for example, served as an organic bridge between positively charged Sn2+ and Ti4+ ions to complete the battery circuit. Ti additionally contributed to the improved structure and stability of the material. Sn contributed its higher theoretical capacity, improving the anode material's electrochemical performance.
Ultimately, the team created a new, inexpensive LIB anode material that maintained a high specific capacity of 345 mAh g-1 at a current density of 1,000 mA g−1 after 700 cycles, which demonstrates the stability of anode material. Scanning electron microscope pictures confirmed that the anode material had no cracks after 700 cycles.
Further analysis of the Sn-Ti-EG anode material revealed that the strong interaction between the Sn and carbon-oxygen species was responsible for the high specific capacity and excellent cyclic stability of the electrode, which may help future researchers design additional anode materials with similar characteristics. The research team sees this latest advance in anode-specific capacity as a stepping stone to additional LIB materials that can improve battery storage capacity and be produced efficiently at large scale.
More information: Yuqing Cai et al, Strong coordination interaction in amorphous Sn-Ti-ethylene glycol compound for stable Li-ion storage, Energy Materials and Devices (2023). DOI: 10.26599/EMD.2023.9370013