This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
EVs that go 1,000 kilometers on a single charge: New gel may make it possible
Futuristic advancements in AI and health care stole the limelight at the tech extravaganza Consumer Electronics Show (CES) 2024. However, battery technology is the game-changer at the heart of these innovations, enabling greater power efficiency. Importantly, electric vehicles are where this technology is being applied most intensely.
Today's EVs can travel around 700km on a single charge, while researchers are aiming for a 1,000km battery range. Researchers are exploring the use of silicon, known for its high storage capacity, as the anode material in lithium-ion batteries for EVs. However, despite its potential, bringing silicon into practical use remains a puzzle that researchers are still working hard to piece together.
Enter Professor Soojin Park, Ph.D. candidate Minjun Je, and Dr. Hye Bin Son from the Department of Chemistry at Pohang University of Science and Technology (POSTECH). They have cracked the code, developing a pocket-friendly and rock-solid next-generation high-energy-density Li-ion battery system using micro silicon particles and gel polymer electrolytes. This work is published in the journal Advanced Science.
Employing silicon as a battery material presents challenges: It expands by more than three times during charging and then contracts back to its original size while discharging, significantly impacting battery efficiency. Utilizing nano-sized silicon (10-9m) partially addresses the issue, but the sophisticated production process is complex and astronomically expensive, making it a challenging budget proposition.
By contrast, micro-sized silicon (10-6m) is superbly practical in terms of cost and energy density. Yet, the expansion issue of the larger silicon particles becomes more pronounced during battery operation, posing limitations for its use as an anode material.
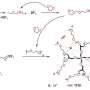
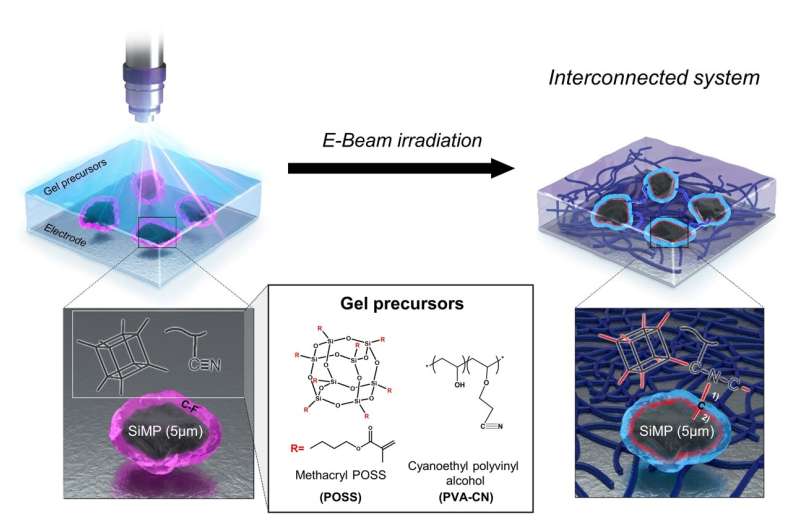
The research team applied gel polymer electrolytes to develop an economical yet stable silicon-based battery system. The electrolyte within a lithium-ion battery is a crucial component, facilitating the movement of ions between the cathode and anode. Unlike conventional liquid electrolytes, gel electrolytes exist in a solid or gel state, characterized by an elastic polymer structure that has better stability than their liquid counterparts.
The research team employed an electron beam to form covalent linkages between micro-silicon particles and gel electrolytes. These covalent linkages serve to disperse internal stress caused by volume expansion during lithium-ion battery operation, alleviating the changes in micro silicon volume and enhancing structural stability.
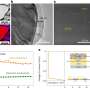
The outcome was remarkable: The battery exhibited stable performance even with micro silicon particles (5μm), which were a hundred times larger than those used in traditional nano-silicon anodes.
Additionally, the silicon-gel electrolyte system developed by the research team exhibited ion conductivity similar to conventional batteries using liquid electrolytes, with an approximate 40% improvement in energy density. Moreover, the team's system holds significant value due to its straightforward manufacturing process that is ready for immediate application.
Professor Soojin Park stressed, "We used a micro-silicon anode, yet we have a stable battery. This research brings us closer to a real high-energy-density lithium-ion battery system."
More information: Minjun Je et al, Formulating Electron Beam‐Induced Covalent Linkages for Stable and High‐Energy‐Density Silicon Microparticle Anode, Advanced Science (2024). DOI: 10.1002/advs.202305298